Abstract
Feather pecking is a serious problem in poultry production. It causes high economical losses and suffering in the affected birds, especially in countries where beak trimming is prohibited. In spite of intensive research, the causes of this damaging behaviour are not fully understood. Most approaches to solve the problem were focused on nutrition and management. All measures have shown only attenuating or no effects on feather pecking. Genetic studies revealed sufficient genetic variation for selection against the damaging behaviour. However, feather pecking is a complex behaviour and more detailed information on the genetic background of the motivation is required to successfully implement this trait in breeding programmes. The prevailing hypothesis explains feather pecking as misdirected foraging behaviour, but other motivations, such as feather eating, aggression, fear and general locomotor activity may be involved. The interrelationships between the above mentioned behaviours have been studied using more than 900 birds of a F2-cross of two lines which have been selected for high and low feather pecking. Heritability, genetic and phenotypic correlations between the traits were estimated using standard statistical models. In addition, structural equation models (SEM) were applied to estimate causal relationships between feather pecking and other traits. Genetic correlation and Lambda coefficients as parameter of the causal link, showed a strong causal effect of feather eating and feather pecking. This supports the hypothesis that feather eating represents a primary cause of feather pecking. There was a substantial causal influence of aggression and general locomotor activity on feather pecking. Open-field activity (fear) and foraging in contrast did not show clear effects on feather pecking.
Keywords
Laying hens, feather pecking, aggression, fear, activity, exploration, genetics
Co-Authors
J.B. Kjaer (FLI Celle), V. Lutz( University of Hohenheim), M. Grashorn (University of Hohenheim), W. Bessei (University of Hohenheim)
Introduction
Welfare has become a major issue in poultry production in industrialised countries. Intensive management systems and selection for production traits are assumed to compromise animal welfare. Examples for the antagonism of performance and welfare-related traits are leg weakness, ascites, sudden death in fast growing broilers (Bessei and Gerken, 2006) and bone breakage in laying hens (Moinard et al., 1998). The discussion has gained momentum through the development of genomic tools which, when applied in commercial breeding, will further increase performance. In the past high performance has been considered as an indirect criterion of welfare. There is however, increasing awareness of the fact that selection for growth or laying rate may lead to welfare problems, and the responsibility of scientists for the wellbeing of farm animals has been expressed in particular by the Precision Animal Breeding framework (Flint and Woolliams, 2007) and the SEFABAR Project (“Sustainable European Farm Animal Breeding and Reproduction”, SEFABAR, 2003). Economic and welfare-related criteria are not necessarily in contrast. Damaging feather pecking and cannibalism for instance, represent severe welfare problems in poultry and have important economic implications. The problem will become more important when beak trimming is banned for welfare reason. Recent experiments with intact-beak birds have shown that management procedures may attenuate feather pecking but do not prevent it. Since feather pecking has a strong genetic component, methods are sought to include this behaviour in selection programmes. Understanding the causes of feather pecking is a prerequisite to solve the problem. In the following we present quantitative genetic analyses of feather pecking and causal relationships between feather pecking and selected behavioural criteria using structural equation models as introduced by Gianola and Soerensen (2004).
State of information and experimental approach
Damaging feather pecking is a complex behaviour. Despite extensive research during the last decades the underlying causes are not known. There exist however various hypotheses in its motivation. The most widespread theory relates feather pecking with feeding and feed searching (foraging) (Blokhuis, 1986; Huber-Eicher and Wechsler, 1998). According to the foraging theory it is assumed that foraging behaviour is redirected towards the feathers of group mates. The foraging theory however, has failed to explain feather pecking in various studies (Hocking et al., 2004; Newberry et al., 2007). Bessei and Kjaer (2015) proposed feather eating as primary underlying motivation. Other motivations for feather pecking which are under discussion are aggression (Bessei et al., 2013), fear (Rodenburg et al., 2010) and spontaneous locomotor activity (Kjaer, 2009). The assumption that feather pecking depends on dust-bathing (Vestergaard and Lisborg, 1993) has not been confirmed in later experiments and is not further considered.
Assumptions on the above mentioned association of feather pecking and other behaviours are mainly based on the comparison of group means and on conventional correlation estimates which do not reveal causal relationships. There exist however, methods such as Structural Equation Models (SEM), to estimate causal effects among traits (Gianola and Soerensen, 2004). Observations of feather pecking (FP), feather eating (FE), aggressive pecking (AP), open-field activity (fear) (OFA), general locomotor activity (GLA) and foraging (FOR) have been observed in a large F2-cross population (> 900 birds) originating from two lines selected for high and low feather pecking (Figure 1). standard multi-trait models (SMS) and structural equation models (SEM) have been used to estimate genetic parameters and to discover causal relationships between the above mentioned behaviours. The application of SEM requires precise a priori definitions of the hypotheses. Therefore, sets of three behaviours each were used. Only behaviours with assumed causal relationships have been chosen.

Feather pecking, feather eating and aggressive pecking
Previous studies have shown that feather pecking and feather eating are closely related. Birds with a high propensity of severe feather pecking of pen mates were found to eat more feathers from plastic dishes when kept individually (McKeegan and Savory, 2001). Laying hens of a high feather pecking line preferred feathers vs. wood shavings in choice experiments and ate more feathers from a plastic dish than hens from a low feather pecking line (Harlander-Matauschek and Häusler, 2009). On the basis of these results we hypothesized that feather eating is the primary motivation which influence feather pecking. It is generally acknowledged that aggressive pecking and feather pecking clearly differ in their phenotypic pattern and are driven by different motivations (Savory, 1995; Bilcik and Keeling, 1999). Aggressive behaviour has proved to be heritable in quantitative genetic studies and selection experiments (Craig et al., 1965).
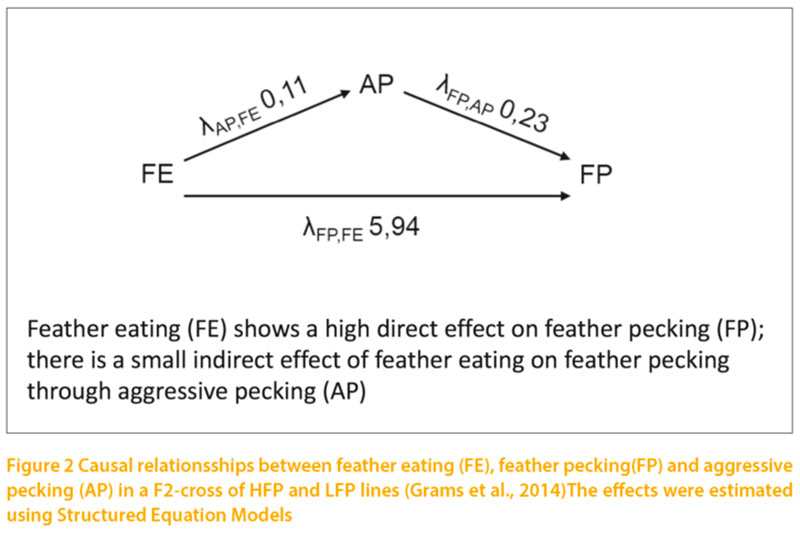
Results have been reported in detail by Bennewitz et al. (2014) and Grams et al. (2014). The heritability estimates for FP varied between 0.11 and 0.20, depending on the statistical model. FE showed considerably higher heritabilities (0.36 – 0.57). There were very high genetic correlations of FP with FE and AP. All phenotypic correlations were positive but on a lower level than the genetic correlations. On the basis of previous information we hypothesised that FE influences FP and AP, and that AP influences FP. The estimated causal effect of FE on FP, λ(FP,FE), was 5.94 (figure 2). For the influence of feather eating on aggressive pecking λ(AP,FE) and of aggressive pecking on feather pecking λ(FP,AP), the λ- values were positive (0.11 and 0.23) but considerably lower than for the effect of feather eating on feather pecking. The results confirmed our hypothesis that FE is the primary motivation for FP. The effect of FE on AP was also low. The high genetic correlation between feather pecking and aggressive pecking and the positive λ(FP, AP) show that the influence of aggression has been underestimated in the past. Though aggression and feather pecking are different in their motoric pattern and the underlying motivation, aggression may reinforce the expression of FP.
Feather pecking, feather eating and general locomotor activity
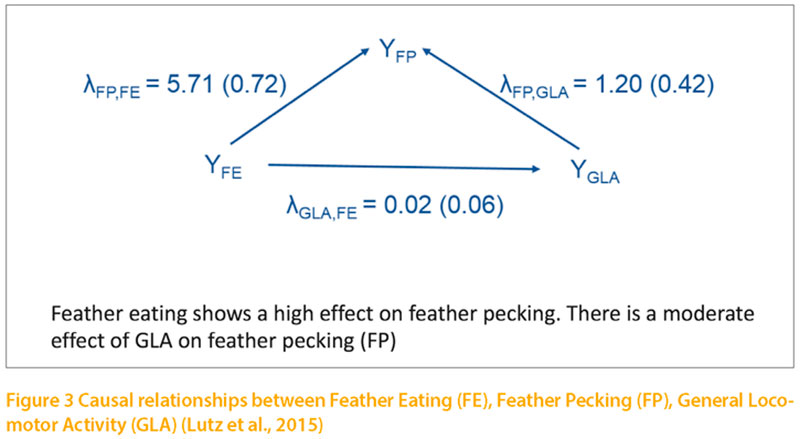
Locomotor activity is usually activated through numerous different motivations, such as exploration, aggression, egg production and flight. As mentioned below, locomotor activity can be inhibited by fear. Under practical conditions it is not possible to determine the underlying motivation. There exist individual differences in the level of locomotor activity of animals which are independent of particular motivations. This “General Locomotor Activity” (GLA) is considered the result of a genetically determined spontaneous activity. Heritability estimates for GLA have been reported by McClearn (1961) in mice and Jezierski and Bessei (1978). Kjaer (2009) selected chicken lines divergently for high and low GLA using the same recording system. He estimated heritability coefficients between 0.20 and 0.30 (pers. communication).The author hypothesised that feather pecking may be the result of a hyperactivity disorder. This hypothesis was supported by physiological studies, which showed that both, feather pecking and hyperactivity are influenced by the dopamine system (Kjaer et al., 2004; van Hierden et al., 2005). Long-time recording of locomotor activity in pullets of the above mentioned F2-cross were used to test this hypothesis (Lutz et al., 2016). At 18 weeks of age locomotor activity was recorded during 9 consecutive days using RFID techniques. The heritability of GLA was 0.29 and both, phenotypic and genetic correlations with FP were positive (0.16 and 0.47). Regarding the causal associations between the traits we hypothesized that GLA influences FP and that FE influences FP and GLA. The results from SEM are shown in figure 3. Structure coefficients revealed a causal influence of GLA on FP (λFP,GLA = 1.20) and of FE on FP (λ = 5.71). The effect of FE on GLA was low. The results confirm the hypothesis that GLA influences FP.
However, the effect of FE as primary motivation on FP is not mediated through the GLA. The effect of GLA on FP is obviously smaller than the effect of FE on FP. Both effects seem to be independent from each other.
Feather pecking, foraging and fear
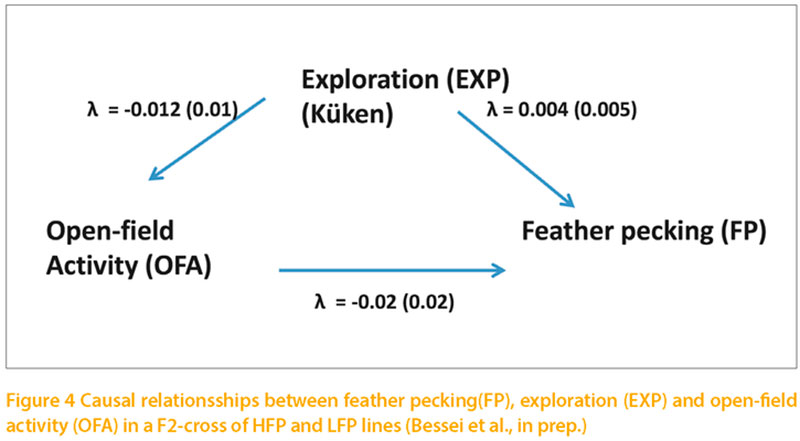
Foraging (FOR) and fear play an important role in the surviving strategies of feral animals. High fear levels inhibit exploration and, thus, exploitation of unknown feed resources. However, fear is essential for survival under high pressure of predators. Hence the foraging activity observed under natural conditions is considered a compromise of exploration and fear. Recent observations have shown that this mechanism still exists in the domestic fowl kept in free range. Hens showing high fear in the Tonic Immobility test spent less time in the free range (Hartcher et al., 2016). There exist numerous tests to assess fear in animals. The open-field test has frequently been used in laboratory animals and in the domestic fowl. Exposing the animals to the open-field situation induces immobility as initial fear response, which is followed by locomotor activity. High open-field activity (OFA) indicates low level of fear and is considered as exploratory activity (Jones, 1989). The open-field test was considered suitable for the present study because of its implication on exploratory behaviour. Moreover, high OFA in young chicks was reported to be predictive for low incidence of feather pecking (Rodenburg et al., 2004). Data of FP, OFA and FOR of the F2-cross of the lines selected for high and low feather pecking have therefore been analysed using SEM. OFA was recorded as number of steps in a 3 minutes test at 7 and 8 days of age. FOR was defined as time spent walking and litter pecking in the home pen during a 20 minutes observation period at 3 and 4 weeks of age. It was hypothesised that OFA and FOR influence FP, and FOR influence OFA. The heritability of FOR was zero. Therefore no genetic correlations could be estimated for this trait. The heritability of OFA was 0.21.
The phenotypic correlation between FP, OFA and FOR as well as the genetic correlation between FP and OFA were close to zero (Grams et al., 2014). All λ-coefficients were close to zero (figure 4).
The results show that the assumed role of foraging and fear on feather pecking as influencing factors for feather pecking have been over-estimated. In contrast to the prevailing hypotheses neither FOR nor OFA showed a relevant causal effect on FP.

References
BENNEWITZ, J., BÖGELEIN, S., STRATZ, P., RODEHUTSCORD, M., PIEPHO, H.P., KJAER, J.B. and BESSEI, W. (2014) Genetic parameters for feather pecking and aggressive behavior in a large F2-cross of laying hens using generalized linear mixed models. Poultry science 93: 810-817.
BESSEI, W. (2013) The effect of selection for high and low feather pecking on aggression – related behaviours of laying hens.
Archiv für Geflügelkunde 77: 10; 10-14; 14.
BESSEI, W. and KJAER, J.B. (2015) Feather pecking in layers – State of research and implications. 26: Proc. Austalian Poultry Science Symposium, Sydney, Australia, 9th – 12th Febr. 2015:214-221.
BILCIK, B. and KEELING, L.J. (1999) Changesin feather condition in relation to feather pecking and aggressive behaviour in laying hens. British poultry science 40: 444-451.
BLOKHUIS, H.J. (1986) Feather pecking in poultry: its relation with ground pecking. Applied Animal Behaviour Science 16: 63-?.
CRAIG, J.V., ORTMAN, L.L. and GUHL, A.M. (1965) Genetic selection for social dominance ability in chickens. Poult. Sci. 13: 114-131.
DAWKINS, M.S. and LAYTON, R. (2012) Breeding for better welfare: Genetic goals for broiler chickens and their parents. Animal Welfare 21: 147-155.
ELLEN, E.D., VISSCHER, J., VAN ARENDONK, J.A.M. and BIJMA, P. (2008). Survival of laying hens: Genetic parameters for direct and associative effects in three purebred layer lines. 87: 233-239.
GERKEN, M. and BESSEI, W. (2006) Welfare in meat type poultry. WPSA – Book of Abstracts XIIth European Poultry Conference, 10 – 14 September 2006 in Verona: 582-?.
GIANOLA, D. and SOERENSEN, D. (2004) Quantitative genetic models for describing simultaneous and recursive relationships between phenotypes. 167: 1407-1424.
GRAMS, V., BESSEI, W., PIEPHO, H.-. and BENNEWITZ, J. (2014) Genetic parameters for feather pecking and aggressive behavior in laying hens using Poisson and linear models. Proc. 10th World Congress of Genetics Applied to Livestock Production, Vancouver, Canada, August 17-22, 2014.
GRAMS, V., BÖGELEIN, S., GRASHORN, M.A., BESSEI, W. and BENNEWITZ, J. (2015) Quantitative Genetic Analysis of Traits Related to Fear and Feather Pecking in Laying Hens. Behavior genetics 45: 228-235.
HARLANDER-MATAUSCHEK, A. and HÄUSLER, K. (2009) Understanding feather eating behaviour in laying hens 117: 35-?.
HARTCHER, K.M., HICKEY, K.A., HEMSWORTH, P.H., CRONIN, G.M., WILKINSON, S.J. and SINGH, M. (2016) Relationships between range access as monitored by radio frequency identification technology, fearfulness, and plumage damage in freerange laying hens. Animal 10: 847-853.
HOCKING, P.M., CHANNING, C.E., ROBERTSON, G.W., EDMOND, A. and JONES, R.B. (2004) Between breed genetic variation for welfare-related behavioural traits in domestic fowl. Applied Animal Behaviour Science 89: 85-105.
JONES, R.B. (1989) Avian open-field research and related effects on environmental novelty. The Psychological Record 39: 397-420.
KJAER, J.B. (2009) Feather pecking in domestic fowl is genetically related to locomotor activity levels: Implications for a hyperactivity disorder model of feather pecking. Behavior genetics 39: 564-570.
LUTZ, V., KJAER, J.B., IFFLAND, H., RODEHUTSCORD, M., BESSEI, W. and BENNEWITZ, J. (2016) Quantitative genetic analysis of causal relationships among feather pecking, feather eating, and general locomotor activity in laying hens using structural equation models. Poultry Science 95: 1757-1763.
MCKEEGAN, D.E.F. and SAVORY, C.J. (2001) Feather eating in individually caged hens which differ in their propensity to feather peck. Journal? 73: 131-140.
MCKEEGAN, D.E.F., SAVORY, C.J., MACLEOD, M.G. and MITCHELL, M.A. (1999) Is development of pecking damage in layer pullets influenced by dietary protein source? British Poultry Science 40: 14-15.
MOINARD, C., MORISSE, J.P. and FAURE, J.M. (1998) Effect of cage area, cage height and perches on feather condition, bone breakage and mortality of laying hens. British Poultry Science 39: 198-202.
MOINARD, C., MORISSE, J.P. and FAURE, J.M. (1998) Effect of cage area, cage height and perches on feather condition, bone breakage and mortality of laying hens. British Poultry Science 39: 198-202.
NEWBERRY, R.C., KEELING, L.J., ESTEVEZ, I. and BILCÍK, B. (2007) Behaviour when young as a predictor of severe feather pecking in adult laying hens: The redirected foraging hypothesis revisited. Applied Animal Behaviour Science 107: 262-274.
RODENBURG, T.B., BUITENHUIS, A.J., ASK, B., UITDEHAAG, K.A., KOENE, P., VAN DER POEL, J.J., VAN ARENDONK, J.A.M. and BOVENHUIS, H. (2004) Genetic and phenotypic correlations between feather pecking and open-field response in laying hens at two different ages. Behavior Genetics 34: 407-415.
RODENBURG, T.B., DE HAAS, E.N., NIELSEN, B.L. and BUITENHUIS, A.J. (2010) Fearfulness and feather damage in laying hens divergently selected for high and low feather pecking. Applied Animal Behaviour Science 128: 91-96.
SEFABAR (2003) Sustainable European Farm Animal Breeding and Reproduction. http://www.effab.info/sefabar.html
VAN HIERDEN, Y.M., KORTE, S.M., RUESINK, E.W., VAN REENEN, C.G., ENGEL, B., KORTEBOUWS, G.A.H., KOOLHAAS, J.M. and BLOKHUIS, H.J. (2002) Adrenocortical reactivity and central serotonin and dopamine turnover in young chicks from a high and low feather-pecking line of laying hens. Physiology & Behavior 75: 653-659.
VESTERGAARD, K.S. and LISBORG, L. (1993) A model of feather pecking development which relates to dustbathing in the fowl. Behaviour 126: 291-308.
WECHSLER, B. and HUBER-EICHER, B. (1998) The effect of foraging material and perch height on feather pecking and feather damage in laying hens. Applied Animal Behaviour Science 58: 131-141.
Acknowledgement
Results of the presented strudies are based on a project funded the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG). The authors acknowledge the technical support of the research station Unterer Lindenhof of the University of Hohenheim.
 Linkedin
Linkedin







