Introduction
Autogenous vaccines play a critical role in the treatment and prevention of diseases in Germany and other EU countries. A similar integrated approach of autogenous vaccines and registered vaccines as complementary concepts is practiced in North America. Despite the generally increasing need for flexible & fast solutions many countries of the global animal health market still rely on registered vaccines only, with at times serious negative consequences for veterinarians and farmers. Based on the existing dual approach plus a third vehicle (MUMS vaccines) in the coming, EU countries are without doubt in an advantageous situation.Lohmann Tierzucht (LTZ) has a longstanding history in the field of autogenous vaccines and during the recent past consequently pursued the pro-active and strategic development of these activities. This article intends to build a bridge between 50 years of “Veterinary Laboratory” history, present activities and an aspiring future.
Success factors of the early Lohmann Veterinary Laboratory
During the 1950s and 1960s, through application of advanced poultry farming concepts acquired in the USA, the historic Lohmann & Co, KG was a key player in transforming the „back-yard farming“ stage of Germany’s poultry production into modern, industrialized farming. Given its pioneer status, Lohmann had no choice but to engage in all relevant aspects of integrated animal husbandry, covering genetics, farm management (including equipment), nutrition, diagnostics and, last but not least, prevention and treatment of diseases.Many of the critical diseases affecting commercial flocks did not exist or were unknown in these early times. The Veterinary Laboratory of Lohmann consequently became a frontrunner in diagnostics & vaccine development and naturally evolved into a pro-active interface between veterinarians, opinion leaders, institutes and universities. The holistic approach of Lohmann with all critical competencies under one roof produced revolutionary solutions to keep birds healthy. As always, successful pioneer work was triggered by creativity, courage to go new ways, intensive knowledge and close connection to the market and customers.
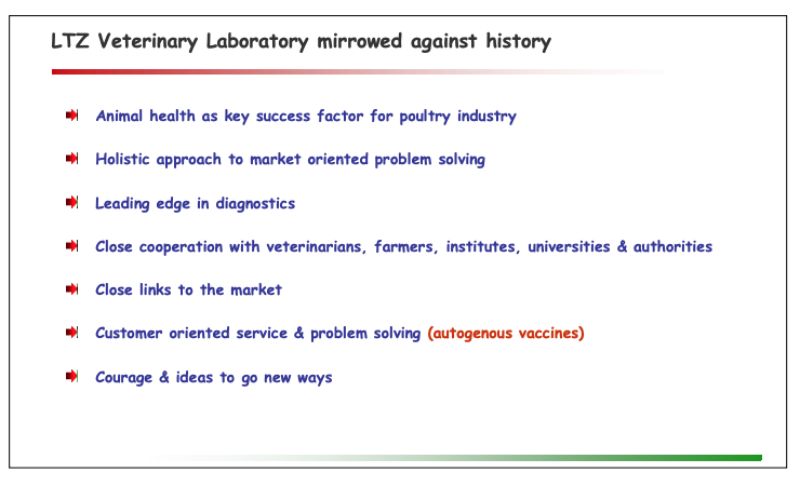
Lohmann Tierzucht “Veterinary Laboratory” mirrored against history
Comparing the activities of today’s “VetLabor” against the background of the past 50 years reveals that all key success factors applied under the historic setup are still in place and practiced today. The only relevant difference is the focus on autogenous instead of registered vaccines.The market of registered vaccines
The landscape of registered vaccines is undergoing significant changes due to several factors:- Declining number of new diseases with „blockbuster potential“ resulting in similar vaccines offered by multiple suppliers, hence increasing competition for standard vaccines
- Market growth in developed major livestock markets limited at best
- Major vaccine players with well-established market shares in livestock focusing on companion animal segment
- Resources & time required for development and registration of vaccines continuing to increase, resulting in rising risks at falling margins
- Producers of registered vaccines under pressure to realize economies of scale, resulting in withdrawals from niche markets and ongoing consolidation
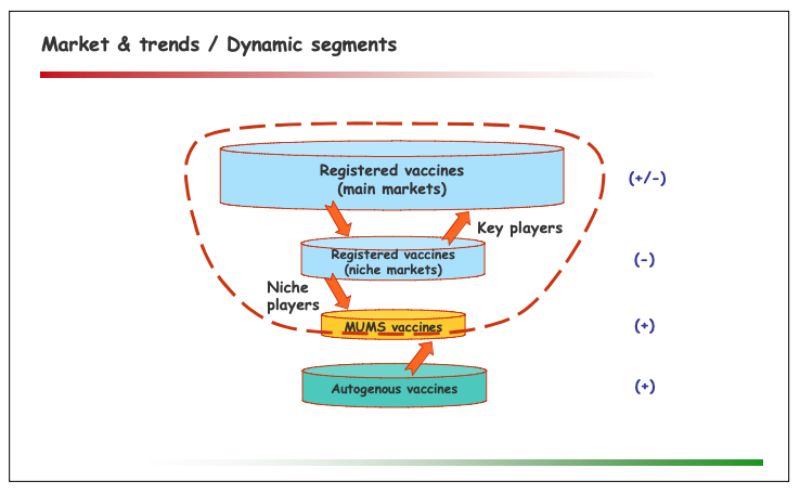
The market of autogenous vaccines
One significant trend in this segment is the growing need for flexible solutions, for a number of reasons: Upcoming new as well as recurring ‘old’ diseases; mutations and resistance among existing strains; changes to practices & priorities in farm management (e.g. increasing integration, free range farming, intensive coverage through veterinarians, cost pressure); improved diagnostics; difficulties of key players to enter or continue serving niche markets.Regulatory perspectives within the European Union
Autogenous vaccines in Germany and the EU are an integral part of modern animal health and hence closely monitored by their respective authorities. An international conference organised in 2003 by the German ‚Paul Ehrlich Institute“ presented the following comments & conclusions in respect to autogenous vaccines:- “Important instrument for prevention of diseases in case of non-availability of registered vaccines”
- “Increasing use in veterinary practice due to changing pathogens, changing farm management and changed priorities of registered vaccine manufacturers”
- “Efficiency is rational for use”
- “Significant changes in structure of poultry industry”
At the same time weaknesses in the regulatory framework have been defined:
- „Unregulated, non-harmonized use of autogenous vaccines not acceptable“;
- „Health of humans & animals has to be considered“
- „Autogenous vaccines cross national borders, thus national regulatory frameworks not adequate any longer“
- „Regulatory framework not in line with present & future needs of animal health“
- „GMP-like production and quality standards desirable“
The conference addressed the topic of „minor use minor species“ (MUMS) vaccines with following conclusions:
- „Commercial pressure forces producers of registered vaccines to focus on major products“
- „Globalisation reduces relative position of niche products“
- „Producers of registered vaccines avoid risk of developing niche products “
- „Lack of suitable registered vaccines leads to Off-Label-Use and illegal use of autogenous vaccines“
- „Development of niche products has to become more attractive“
The evolving picture concerning autogenous & MUMS vaccines was presented as follows:
LTZ investment in state-of-the-art production facility
The changes described above facilitated a pro-active re-orientation of the autogenous vaccine activities of Lohmann Tierzucht, as shown in the following chronological overview of developments:2003-2007 Increasing pressure from authorities to improve production and quality control conditions at the Veterinary Laboratory
2005-2007 Evaluation of several scenarios ensuring a future oriented continuation of existing activities
2007 Decision to invest in new GMP standard production facility, hence creation of first-class conditions for consequent further development of autogenous vaccines
2008 Completion & start-up of new facility
2009 GMP certification and further organisational developments
Vision
Lohmann Tierzucht has a clear vision concerning the future of its activities with autogenous vaccines. Cornerstones of this vision are:- Preferred partner of German poultry veterinarians & farms concerning autogenous vaccines
- Europe-wide accepted partner for poultry diagnostics & autogenous vaccines
- Holistic problem-solving through team competence in critical aspects of poultry health (genetics, management, nutrition, diagnostics, vaccination)
- Dynamic & flexible portfolio with high degree of special products
- Future oriented production facility & quality standards
- Pro-active interface between science & practice
Product portfolio
A portfolio of autogenous vaccines by its very nature is flexible and continuously adapted to market requirements. The portfolio overview outlined below consequently has to be seen as an inventory at this point of time: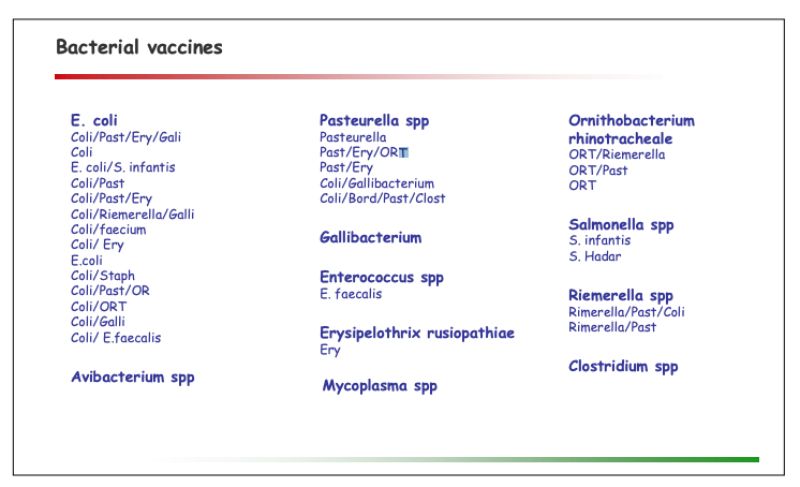
Profile of strength
In a nutshell, autogenous vaccines developed, produced and marketed by Lohmann Tierzucht are the result of 50 years history consequently shaped into a unique profile of strength in the field of autogenous vaccines:- GMP certified production & quality control standards
- Flexibility of small production scale
- Wide range of bacterial & viral vaccines
- Leading competence in determination of field strains
- Worldwide monitoring of disease trends in the field
- Comprehensive competencies in genetics, management, nutrition, diagnostics, vaccines
Zusammenfassung
Bestandspezifische ImpfstoffeStallspezifische Impfstoffe spielen in Deutschland und der EU eine wichtige Rolle in der Behandlung und Prävention von Krankheiten. Während die Notwendigkeit nach flexiblen & schnellen Lösungen in der täglichen Praxis der Tiergesundheit zunimmt, unterliegen registrierte Impfstoffe globalen Trends, die das Bereitstellen von Impfstoffen für Nischenmärkte immer schwieriger machen. Die nationalen Tiergesundheitsbehörden haben diese Entwicklung seit Jahren erkannt und arbeiten gezielt daran, praxisorientierte Rahmenbedingungen zu schaffen. Im Vordergrund stehen hier die verbesserte Kontrolle und EU Harmonisierung von stallspezifischen Impfstoffen sowie die Einführung der neuen Impfstoffkategorie “Minor-Use-Minor-Species (MUMS)”. Dem Markt stehen somit drei sich ergänzende Impfstoffkategorien zur Verfügung. Das Veterinärlabor der Lohmann Tierzucht GmbH blickt auf eine 50jährige Geschichte und bedeutende Pionierleistungen in der Diagnostik und Entwicklung/Herstellung von Impfstoffen zurück. Die enge Einbindung in alle relevanten Aspekte der Tierzucht und die über Jahrzehnte gewachsenen Kompetenzen in der Tiergesundheit geben dem heutigen Veterinärlabor ein ausgeprägtes Stärkeprofil als Partner für Problemlösungen mit stallspezifischen Impfstoffen. Die im September 2008 fertiggestellte Investition in eine neue Produktionsstätte für bestandspezifische Impfstoffe auf GMP Niveau stellt einen konsequenten und wichtigen Meilenstein für die strategische Weiterentwicklung dieses Geschäftsbereichs dar.