Rudolf Preisinger
Professor Dr. Rudolf Preisinger is Senior Group Advisor Breeding & Biotechnology of the EW Group GmbH. He supervises the global research activities associated with layers with special focus on biotechnologies. His focus lies in the research and development and the successful application of new scientific findings in commercial poultry farming. His scientific contributions are documented in more than 250 publications in peer-reviewed journals.
INTRODUCTION
Since January 1st, 2022, the killing of day-old chicks has been prohibited by law in Germany and other European countries like Austria, France and Italy are following. Retailers and consumers are forcing the industry to stop a worldwide used practice.
Due to a high negative correlation between reproductive and growth traits, specialized hybrid breeding developed in the mid-20th century offered a great opportunity for the commercial poultry industry: While broiler lines bred for meat yield reached the desired slaughter weight in an increasingly shorter time with less feed, laying hybrids specifically selected for high laying performance and egg quality achieved large increases in lifetime egg production.
As specialization progressed, male chicks f rom these lines lacked economically viable use. It became common practice worldwide to kill them as day-old chicks. It is quite common in Europe to use day old males as a high-quality feed for zoo animals after CO2 gassing and deep-f reezing.
Increasing ethical and legal concerns about the common practice of killing day-old chicks, which was practiced not only by conventional egg producers but also for the most part in laying hen production according to organic guidelines, brought the idea of sex determination in the egg back into the focus of research (Klein et al., 2003). Sperm-sexing – which is used as a tool for avoiding offspring of the undesired sex in farm animals like cattle – cannot be used in poultry, since in birds, unlike in mammals, the sex of the offspring is determined by the female germ cell.
While automated sex determination still seemed a long way off in the early 2000s (Preisinger, 2003; Phelps, 2003), intensive research and development work has brought at least three proven methods to practical maturity and application since 2020 at the latest (Richard, 2020)
The development of these and other methods as well as the challenges to be overcome on the way f rom an idea to practical application will be presented in the following, completed by case studies and an outlook.
KEY ISSUES AND CHALLENGES
Kaleta and Redmann (2008) specified requirements for an ideal method for in ovo sexing of hybrid lines of layer type chickens:
1 Methods must be applicable to eggs prior to setting for incubation.
2 Methods must not affect the integrity of egg-shells and the interior of eggs.
3 Eggs containing male blastoderm cells need to be used for reasonable purposes.
4 Applied methods should have no negative effects on embryonic development.
5 Rate of hatchability of chicks must not be affected.
6 Necessary technical equipment must be available and its use economically feasible.
7 Methods must be applicable to large numbers of eggs.
8 Sorting by gender must be possible within short periods of time.
9 Methods must be f ree of any negative effect on female reproduction.
10 Post-embryonic physical and behavioral development of chicks remains unchanged.
11 Methods must be acceptable f rom a humanitarian and ethical point of view.
All development approaches have been and are currently still being measured according to these criteria, with many challenges to be overcome on the way f rom an idea to practical application.
In basic research, the first task is to find a “source of information” that provides reliable information about the sex of the embryo as early as possible. A biologically reliable and comprehensible identification of sex-specific distinguishing features (levels of male or female sex hormones, different DNA of the sex chromosomes, sex-typical biomarkers or spectral differences) provide starting points for further developments.
During the research process it is important to check various aspects for later practical application: Are the individual steps required suitable for automation? Can a damage to the embryo and/or negative impact on hatchability be ruled out? Can the information and measurement data obtained be easily interpreted and are they reproducible? Is the respective methodology socially acceptable? Above all, automation, and verification in extensive hatchery trials over the entire incubation period are the most important steps in this phase.
A successful system also must fit into the political and societal requirements and its costs of rearing the roosters must be compared.
TECHNOLOGIES IN COMMERCIAL USE
Three methods for in ovo sexing have successfully finished followed the development phase and reached practical application in Europe. Some of them have been used in commercial hatcheries since 2019.
To date the highest amount of “in ovo chicks” – i.e., female layer chicks without killing the brother – is produced with the hyperspectral analysis system “CHEGGY” (AAT, 2021), followed by endocrinological sexing “Seleggt” (SELEGGT, 2022) and DNA analysis “PlantEgg” (PlantEgg, 2021).
These practically applied methods, their development, and their special characteristics are described in the next pages.

Hyperspectral analysis
The sexual dimorphism in plumage color in commercial brown laying hybrids can be used by the method of hyperspectral analysis for sex-specific sorting of embryos. Hyperspectral analysis is used to generate three-dimensional images.
In brown laying hybrids with sex-specific feather color, sex differences in pigmentation levels are already visible during embryonic development. Göhler et al. (2017)investigated the use of hyperspectral analysis between day 11 and day 14 of incubation and achieved an accuracy of up to 97%.
The analysis can be performed non-invasively through the intact eggshell because the eggshell does not form a barrier for the light spectra captured by the hyperspectral camera. Thus, cross contamination and negative influences on the development of the embryos, the hatching rate and later performance and health of the laying hens are not to be expected.
The process steps of this method include:
1 llumination of the eggs in a measuring chamber from below
2 Detection of the air chamber with a thermal imaging camera
3 Recording of the spectra by the hyperspectral camera from above
4 Analysis of the recorded spectra with a specially developed algorithm
5 Classification and marking of the hatching egg
The entire process can be relatively easily automated and integrated into the routine process in a hatchery. The hatching eggs are loaded to the machine on the regular trays. Automated in this way, the sexing capacity is up to 20,000 hatching eggs per hour.

Already since 2020, the “CHEGGY” system developed by Agri Advanced Technologies (AAT), Visbek, Germany is being used in large-scale hatcheries in several European countries (Zootecnica 2021, AAT 2022).
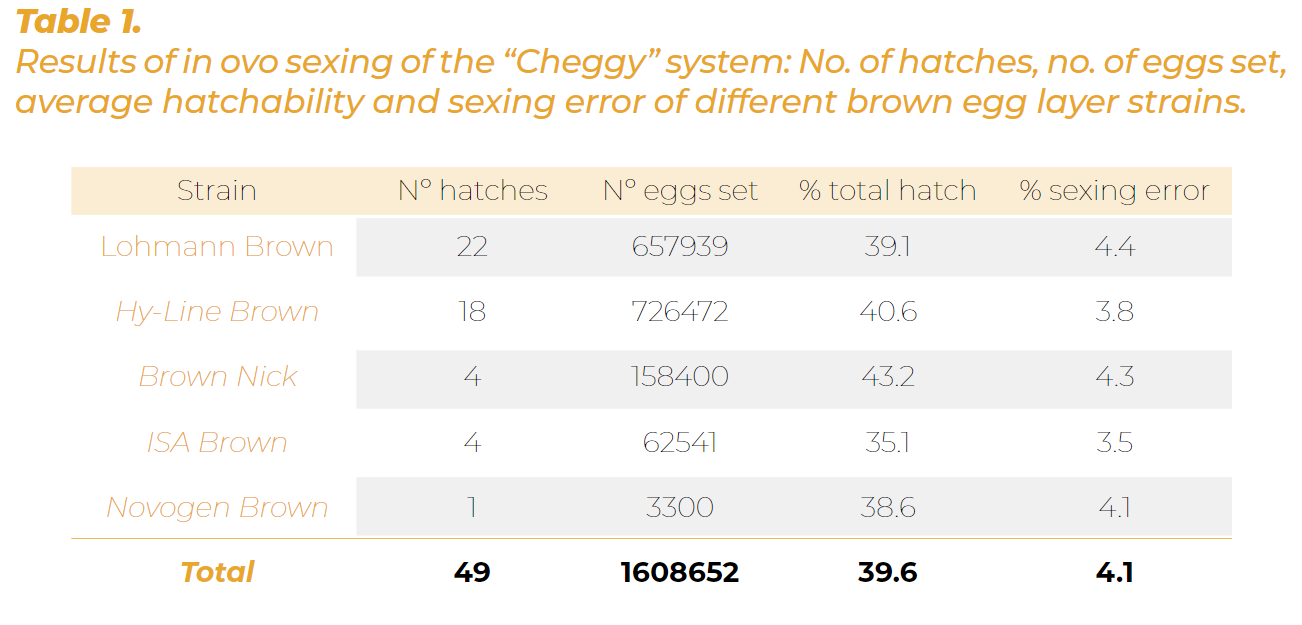
In a recent study using CHEGGY (Förster et al., 2022) the accuracy of sexing was > 95% with an average sexing error of 4.1%.
The impact of the sorting technology on hatchability was 2-3 percentage points lower than the expected hatchability rate without in ovo sex determination. Thus, only about 8% more hatching eggs were needed to produce the targeted number of in ovo-sexed female chicks (Table 1).
Endocrinology
Since about 2010 efforts have been made to detect sex-specific hormonal concentration differences in samples f rom allantoic fluid before 11 days of incubation (Weissmann et al., 2013). Allantoic fluid was manually collected f rom eggs incubated for 7 to 10 days, closely following the method described by Phelps (2003)when eggs have been sampled between 13 and 18 days of incubation.
The fluid was analyzed with an Elisa assay for the levels of estradiol (E2), estrone sulfate (E1S), and testosterone (T), for which sex-specific differences in concentrations are expected in female and male embryos.
On day 7, only E1S was present, but in similar amounts in both male and female embryos. Significant differences were not apparent until day 9, and precise sex identification was possible on day 10. (Figure 1).
Using advanced sampling techniques of allantoic fluid, an accuracy of 98% was achieved in further trials (Weissmann et al., 2014). 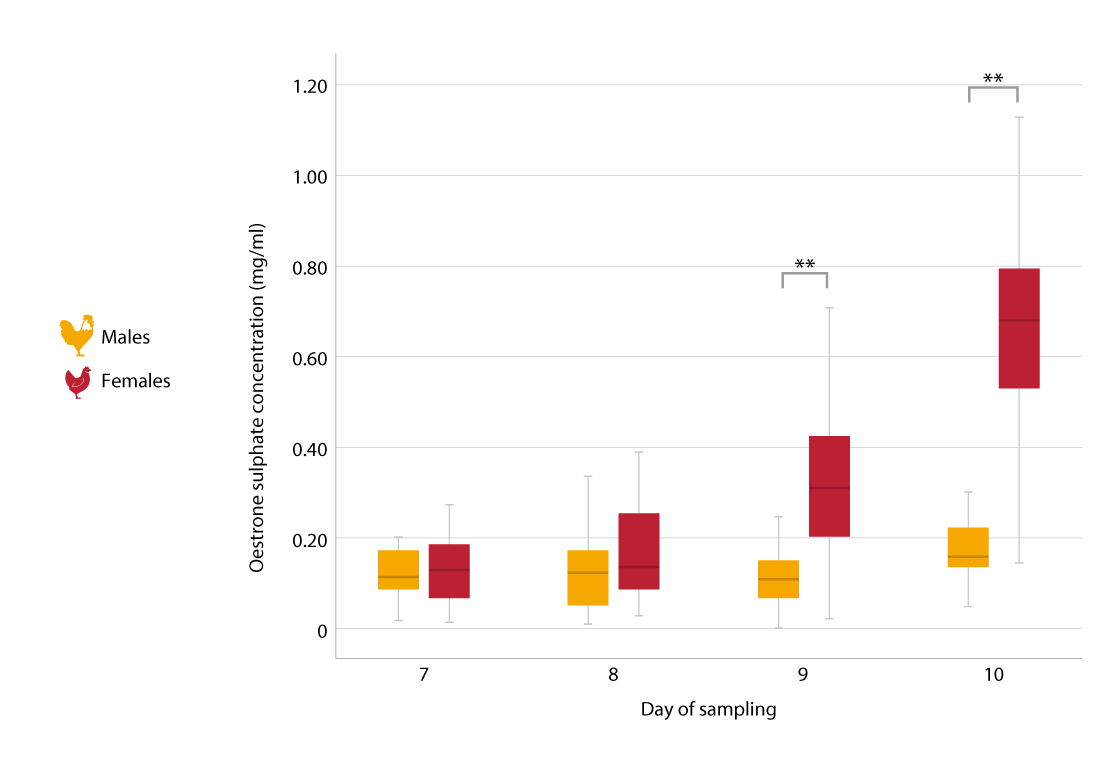
Figure 1. Box plot diagram of estrone sulfate concentrations (ng/mL) of male (green) and female (red) embryos in the allantoic fluid on incubation day 7 to 10. Highly significant differences (p 0.001) are indicated by double asterisks (Einspanier, 2013).
These investigations, which were part of a research project funded by the German Federal Ministry of Food and Agriculture, and the promising results led to the involvement of a leading food retail company, which, together with a project partner, aimed to automate the process and commercialize the system.
Since 2019, a fully automated process is available that carries out the process steps shown in Figures 2 and 3 (next page). According to the manufacturer, 3600 hatching eggs/hour and system can be sorted in this way.
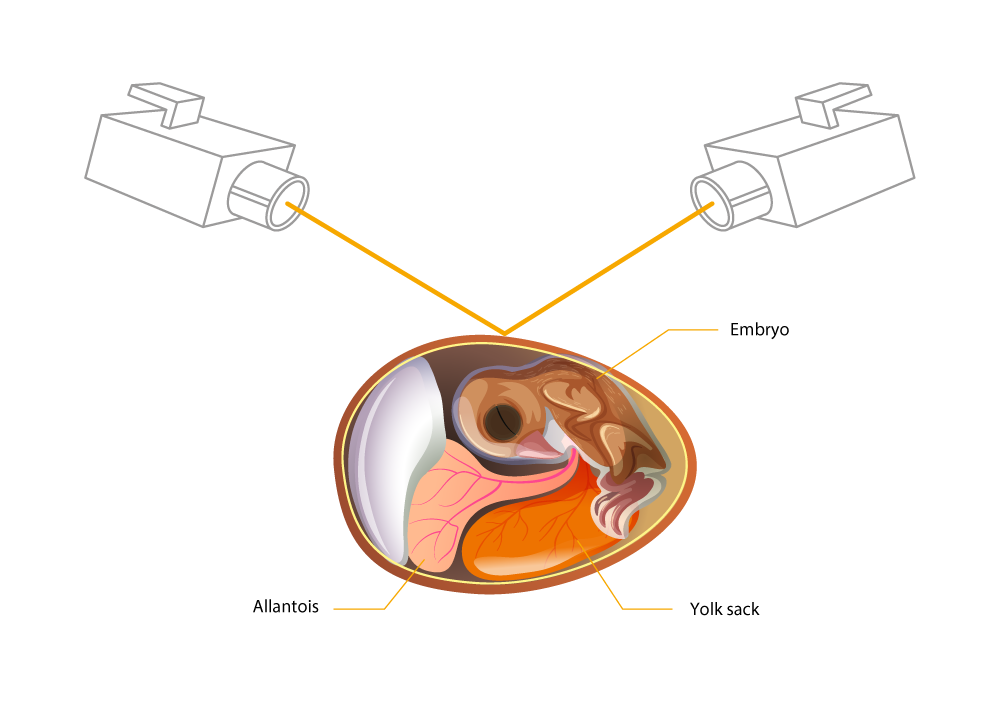
Figure 2. Generating a hole at a defined point with laser.
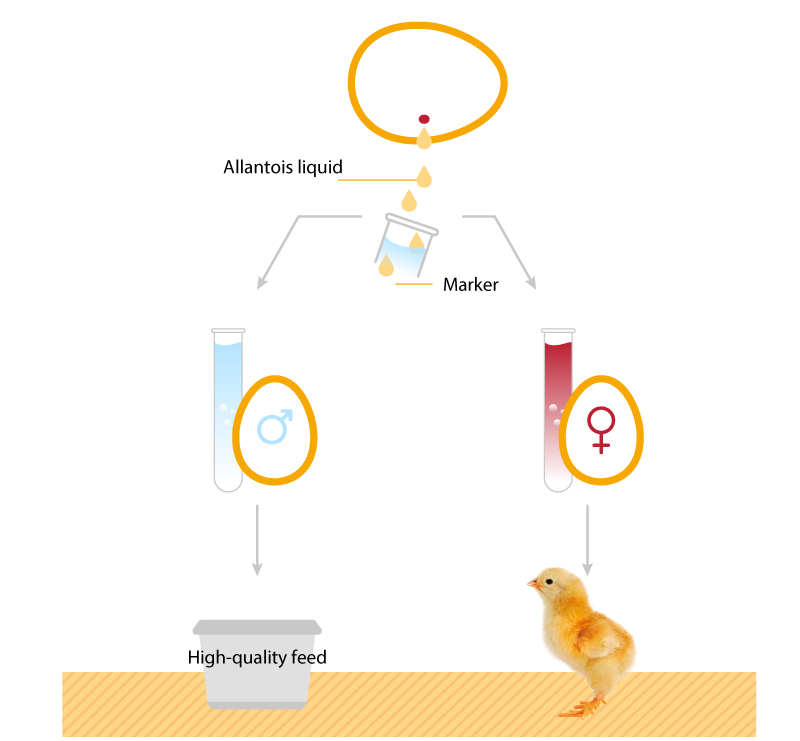
Figure 3. Removal of a small amount of allantoic fluid – analysis of the estrone sulfate content with robot support (Seleggt, 2022).
DNA Analysis
Using the PCR method small amounts of genetic DNA available in the allantoic fluid can be collected and amplified by PCR.
After amplification of the DNA an optical signal is visible indicating the female DNA. In case of male DNA no signal is visible. Thus, a reliable distinction between male and female eggs at an early stage of the incubation is feasible.
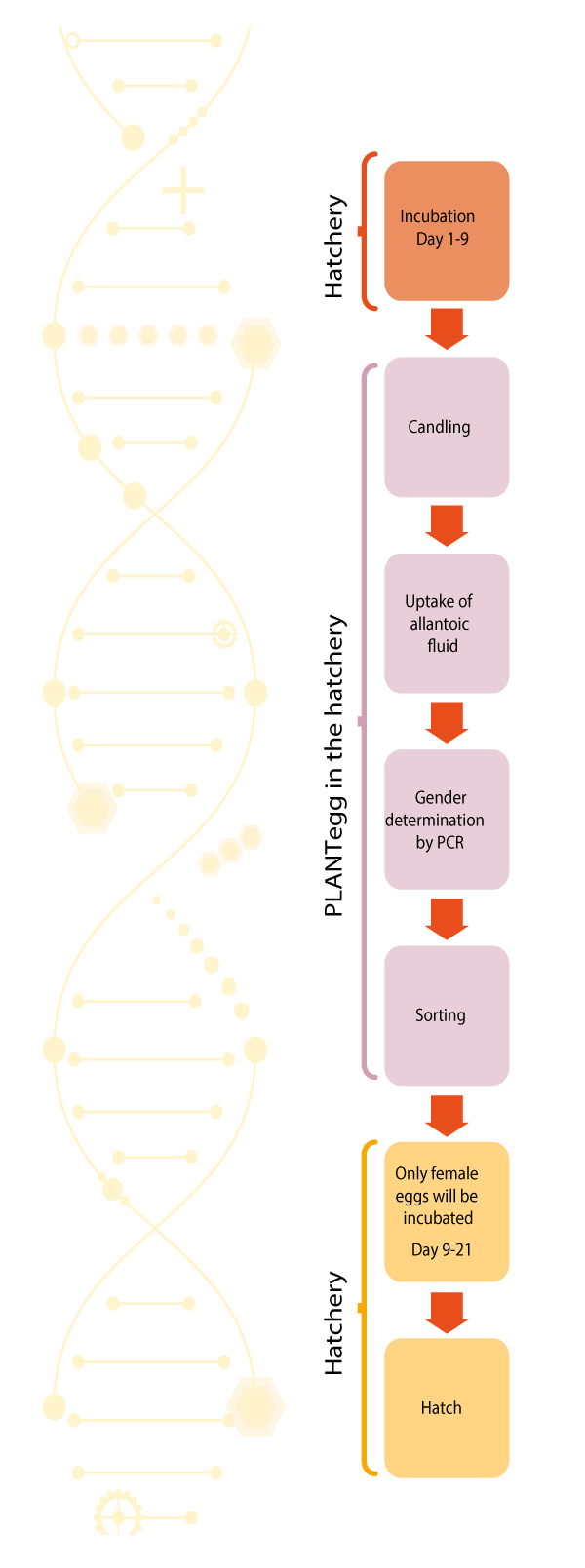
Meanwhile the PLANT egg process is used commercially in hatcheries with a sexing precision of more than 98%. The different steps of the PLANTegg process within the hatchery are explained below and shown schematically in Figure 4.
Figure 4. Different steps of the PLANTegg process (Kleine, 2022).
1 Tu búsqueda no ha generado buenos resultados. Processing: The hatching eggs are candled and only fertilized eggs are transferred at day 9 to a roboting device, which has been installed within the hatchery. A laser beam creates an opening of 0.3 mm diameter in the hatching egg and a pipette retrieves a few drops of allantoic fluid sufficient for the gender determination. 3600 eggs per hour can be processed with one device. The sampling technology is identical with the procedure used by Seleggt (2022).
2 PCR analysis: The allantoic fluid is collected in test tubes and transferred to an extra lab room within the hatchery or to an external container lab attached to the hatchery. There, the allantoic fluid is combined with the PCR reaction chemical. The tubes are finally transferred to the PCR machine and the results (male/female) are generated after about one hour processing time.
3 Sorting: The result for each setter tray and location is transferred electronically to the hatchery and sorting of the male and female eggs takes place in an automated device. Female eggs are returned to the incubators, male eggs are sorted out and used for feed purposes.
The PLANTegg system will be installed within the hatchery and operated by PLANTegg. The modular set up of the equipment – robotic sampling device(s), PCR lab with PCR-machine(s) – can be modular adapted to the capacity of the hatchery.


Mass spectrometry
According to In Ovo, the concentration of glucose, choline and valine in the allantoic fluid is higher in females than in male eggs and could thus serve as biomarkers (Bruins and Stutterheim, 2014).In Ovo, a spin-off of Leiden University, identified a single biomarker that can be detected by mass spectrometry in a fast and high throughput device.
The sampling for allantoic fluid is based on syringe which penetrates the eggshell and collects the liquid for further processing. Large scale field results regarding throughput and accuracy are not available yet.
ADDITIONAL RESEARCH PROJECTS
Figure 5. is showing an overview over research projects world-wide – a snapshot without claiming completeness. Patents have already been disclosed or granted for numerous process inventions to solve the problem of early sex determination in eggs and “breakthroughs in early sex determination” have been announced in press releases.
However, f rom the available documents it is usually not possible to determine to what extent the respective inventions could be successfully implemented in commercial hatcheries.
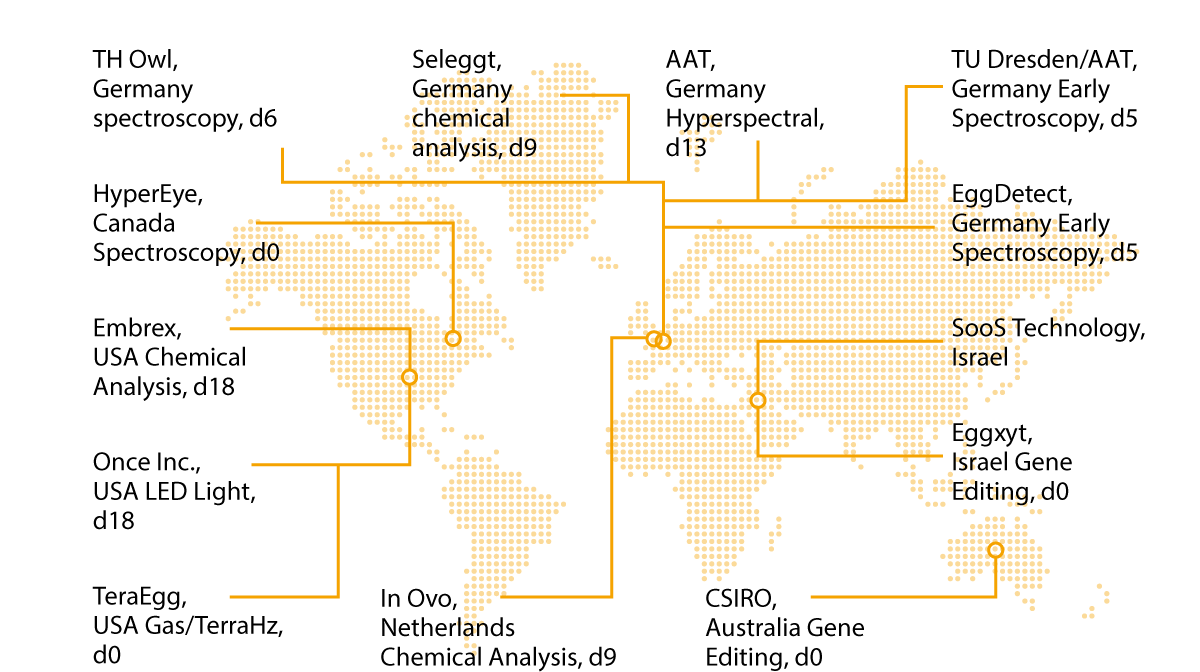
Figure 5. Research projects and approaches for in ovo sex determination world-wide.
There is also often a lack of scientifically proven information on the methodology, diagnostic accuracy and other relevant aspects (precision, speed, hatching rate, operational costs, animal health and performance parameters of the hens, etc.) (Krautwald-Junghanns et al., 2018).
This applies, for example, to the “Hypereye” method developed in Canada, which is supposed not only to allow fertility control but also sex determination by means of hyperspectral imaging on the unincubated egg.
The US company ONCE claims to have developed a “genetic switch” that can be used to selectively activate or deactivate light absorption centers in sex-determining genes using narrowband light-emitting diodes. In this way, it should be possible to selectively produce only offspring of the desired sex (females in laying hen production, males in broiler production). SOOS, a company located in Israel, follows a similar approach of turning genetically male embryos into functional female chicks.
According to its own information, this is done non-invasively during the incubation process by applying sound waves and other specific incubation parameters. Unfortunately, no reliable results have been published yet, and no information is available concerning trials or practical use of those methods.
But there are more approaches with more chance of success, which are described below.
Spectroscopic methods
The spectroscopic examinations are non-invasive in the sense that no tissue has to be collected.
Raman- and Fluorescence spectroscopy
(Figure 7.)
It is advantageous to uncover the blunt egg pole, as the inner shell membrane remains intact. The latest generation of the method, which was primarily developed at the Technical University (TU) Dresden, Germany, uses two laser beams of different wavelengths. The scattered light f rom the hemoglobin is used to identify the sex in a combination of Raman and fluorescence spectroscopy. While Raman spectra provide molecular structural features, fluorescence primarily provides information on the concentration of hemoglobin (Galli et al., 2016, 2017a-c).
(Figure 8.) Male eggs can be sorted out and processed further, female eggs are resealed with a medical plaster and incubated until hatching.
This highly demanding process could not yet be scaled up beyond the laboratory level. In larger and more extensive trials in hatcheries, the commercial partner of TU Dresden, Agri Advanced Technologies (AAT) has not yet been able to fully reproduce the published lab scale results. Further trials should bring this application to market maturity with a consistently high level of accuracy and speed.
Time-resolved laser-induced fluorescence spectroscopy
Magnetic resonance imaging
Gene editing
The female chicks hatching f rom such mating do not show any genetically modified genes according to current knowledge.

cf. https://www.eggxyt.com/
Avian egg odor
Specifically, it was shown that egg volatiles undergo change over the course of incubation, and differ not only between fertile and infertile eggs, but were also sex-predictive as early as day 1 of incubation.
One of the volatile ketones (2-undecanone) which differed between eggs containing male and female embryos has previously been identified as a hormone-linked constituent of avian odor (Whittaker et al., 2011).
They also found sex-specific differences in the composition (or content) of volatiles between (hatched) male and female chicken eggs during the first 9 days of incubation and between unhatched male and female chicken eggs of different lines.

DISCUSSION
Globally The development of applicable, reliable and cost efficient procedures could lead to the avoidance of the killing of male layer hybrids in the future, thus making a valuable contribution to animal welfare in commercial egg production.
The lack of profitability of rearing males f rom layer strains results, among other things, f rom their longer fattening period, a slow growth rate with higher feed consumption and especially a lower proportion of breast meat, which is particularly appreciated by consumers.
For consumers, there is also the challenge of maintaining an overview on all available options. In ovo sexing has the potential to end the culling of male chicks in the egg-producing industry.
However, to meet industry requirements for precision and speed of procedures, full automation of the processes is required to make this technology applicable in hatcheries (Galli et al., 2017b).
However, such breeding lines, which are already commercially available, still show significantly lower economic performance (lower egg numbers and smaller egg size, higher feed consumption with longer growing period, smaller breast meat yield than the specialized layer or broiler lines respectively (Icken et al., 2013).
Despite consumer acceptance, only a very specialized market segment can be served with dual-purpose chicken products at this time (Brümmer et al, 2018; Gangnat et al, 2018; Lambertz et al, 2018).
Twenty years ago, the challenges for in ovo sex determination were more of a biological and technical nature; today, it is driven by political and ethical challenges.
In Germany and other European countries, there are currently (at least) three alternatives on the market that can successfully identify the sex of an embryo during the second trimester of incubation.
Organic farming associations prefer rearing of the males and in some cases completely reject in ovo sex determination. Consumers now have the choice to buy eggs f rom supply chains “without chick culling”, but often do not exactly know if the laying hens’ brothers were raised or if an in ovo sexing process was used.

Nociception in chicken embryos
In this context, the question arises, at which age domestic chicken embryos are capable of nociception and can therefore be considered sentient. The capacity of avian embryos for nociception is currently no longer in question (Aleksandrowicz and Herr, 2015; Bjørnstad et al., 2015). A prerequisite for sentience is the presence of a central nervous system and functional pathways that allow conduction of stimuli to the brain. The development of the central nervous system in the chicken embryo is a dynamic process that begins on the 2nd day of incubation and matures continuously until hatch.
Currently, in the absence of reliable data, it is generally assumed that the chicken embryo is not yet capable of nociception before the 7th day of incubation, while the ability of pain perception is likely to be present f rom day 15 on.
Since all currently available techniques for gender sorting are applied exactly in this period, the question arises whether the rejected male eggs can be handled in an animal welfare friendly and consumer accepted way. The use of electrical current flow was investigated as a method of anaesthesia of chicken embryos to avoid a possible perception of pain when the incubation process is terminated (Zumbrink et al., 2020).
Based on the promising results of this study, a high throughput system for electrical anaesthesia (“Stunny”) was constructed by AAT. The stunning unit in combination with hyperspectral in ovo sex determination (or other techniques) can be seen as a bridge technology.
It provides an important step in the animal welfare discussion about chick culling, until techniques for the determination of sex are applicable at an earlier stage of embryonic development (Zumbrink et al, 2022).
Outlook
The further development of the described spectroscopic techniques as well as other approaches is needed for solutions that reliably perform as early as possible during incubation. Much hope lies in this technical progress since the alternatives – rearing of the males or use of dual-purpose breeds – are neither sustainable nor environmentally f riendly and can only be a solution for niche markets.
Note: This article is based on a more detailed review, (CH07 – In ovo sexing in poultry chicks), to be published in: French, N, (ed.) (2023), Understanding embryo development and improving hatchery practice in poultry production, Burleigh Dodds Science Publishing, Cam bride, UK.

cf. https://www.eggxyt.com/