Isabelle Ruhnke graduated in Veterinary Medicine from the Freie Universität Berlin, Germany, and performed work for her doctoral thesis on glucose transport and gastrointestinal diseases at the Gastrointestinal Laboratory at Texas A&M University, USA. Her PhD on the impact of feed technologies and feed particle size on broiler chickens and laying hens was completed at the Institute of Animal Nutrition, Freie Universität Berlin. After 8 years of applied research on nutrition, health and welfare of chickens became her passion, so Dr. Ruhnke decided to dedicate her research to the nutritional management of free-range laying hens at the University of New England, Australia. Isabelle is now the Head of Poultry Division of the Department of Veterinary Medicine at Freie Universität Berlin, Germany.
Isabelle.Ruhnke@fu-berlin.de
ABSTRACT
The internal and external quality of table eggs is of crucial importance to the egg industry and the consumer, as a variety of markets have different demands. To meet this demand, several housing systems have been established, which allow for different breeds and strains to generate their eggs. This article
summarises common challenges seen in various housing systems and shares information that can help to overcome typical pitfalls. Housing system has no impact on egg shape but inconsistent information has been obtained on egg weight, egg shell breaking strength, shell thickness, cracked eggs and dirty eggs. An impact on shell colour could be observed where free-range hens had lighter shell colour compared to barn and caged hens. The housing system may affect food safety and egg quality especially due to challenges associated with nest box use as well as ingestion of dioxin and lead contaminated soil, but does not necessarily impact the Salmonella
prevalence as all housing systems have the potential to produce eggs of inferior external and internal quality depending on how they are managed. Furthermore, special emphasis is provided on new insights into the impact of freerange flock sub-populations on laying performance and egg quality.
While egg production differs with range usage, egg quality shows little difference between ranging and non-ranging hens. Awareness of typical pitfalls associated with the different housing systems and their impact of egg quality allows for prevention and preparedness, resulting in the reliable outcome of immaculate and safe eggs with minimal losses and hence improved sustainability of the industry.
INTRODUCTION
It has been estimated that approximately 6.5 billion hens are used worldwide for egg production (Windhorst et al., 2013). Assuming 320 eggs/hen/year with an average weight of 65g/egg, this would result in an annual production of 2.080 trillion eggs and an egg mass of 135.2 million tonnes. The internal and external quality of eggs is of crucial importance to the egg industry and the consumer, as various markets have different demands. To meet this demand, several housing
systems have been established, which allow for different breeds and strains to generate their eggs. For example, Asia houses the world‘s largest percentage of laying hens (61.3%; 2.9 billion), where >80% of these are kept predominantly in conventional cage housing systems. In contrast, the second largest egg producer, the European Union (including the UK), houses approximately 400 million laying hens with only 50.4% of these hens kept in (enriched) cages (Eurostat, 2011; Windhorst 2013). While climatic opportunities, resource availability, retailer marketing strategies and the consumers’ perception about hen welfare influence the producers’ decision on farming a specific housing system, the consumers’ perception about egg quality is also relevant. In a survey among 345 consumers in the UK, more than 50% believed that there is a difference in egg taste depending on the housing system and 35% believed that taste is the main distinguishing feature
between cage and free-range eggs (Parrot, 2004).
However, the external and internal egg quality is impacted by multiple factors including hen breed or strain, epigenetics, pullet quality, light intensity, duration and wavelength, hen age, body weight and body condition, the nutritional status of the hen, the current diet, drinking water quality, stress including heat stress, housing and management as well as the disease status of the flock (Figure 1; Aerni et al., 2005; Englmaierova et al., 2014; Lordelo et al., 2016; Roberts, 2004; Roberts, 2008; Singh and Cowieson 2013; Tumova et al., 2003; Van Horne 1996).
Factors that impact egg quality. In the majority of cases, compromised egg quality is multifactorial.
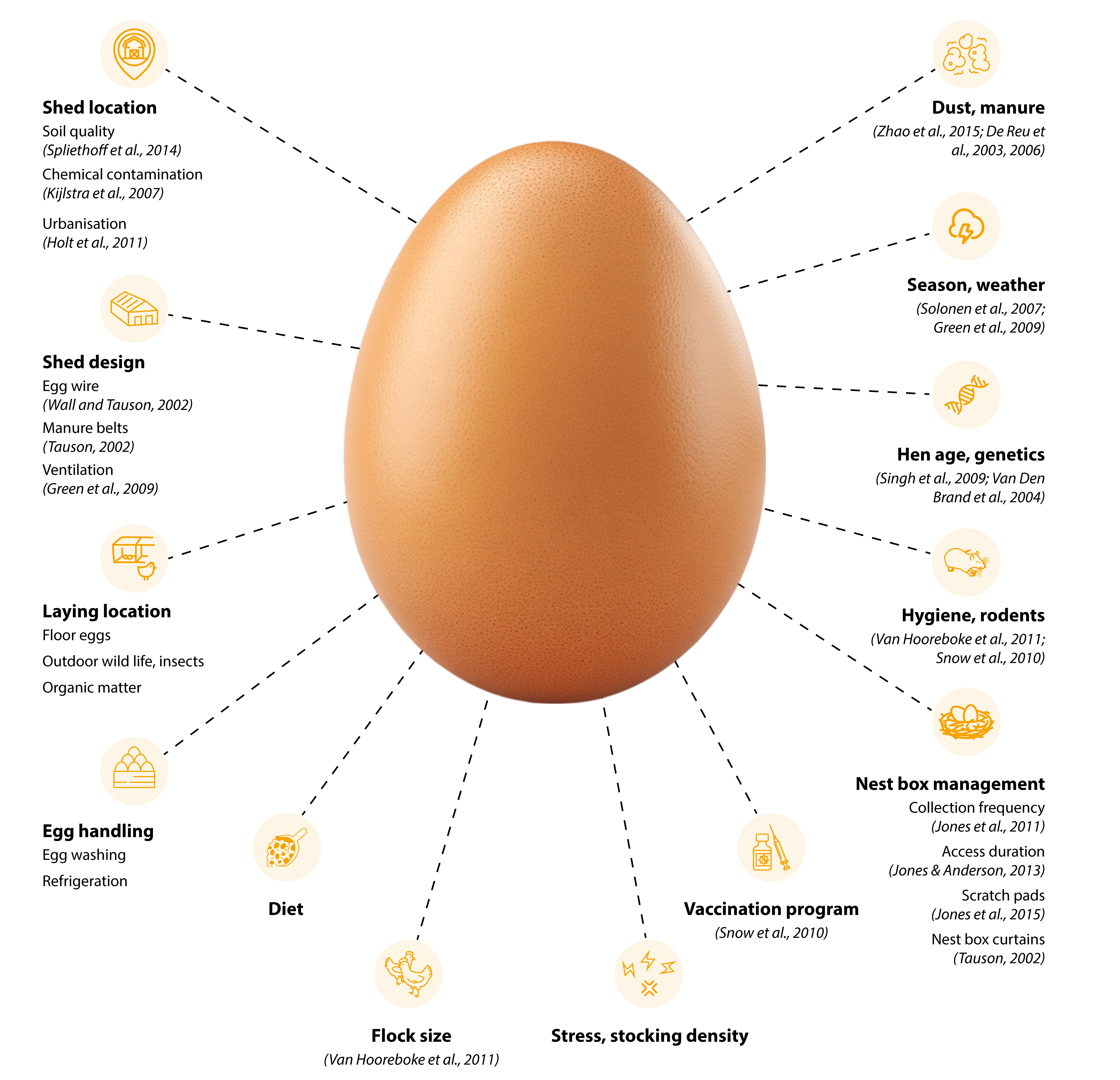
In addition, various interactions such as genotype and environment should be considered and, while many factors mentioned above may be challenging to control (weather, shed location, hen age etc.), others can be manipulated easily (management including egg handling and vaccination) to achieve the desired outcomes (Singh et al., 2009). Poor egg quality does not necessarily mean that all hens in a flock produce eggs of reduced quality, but rather that the variability within the flock increases (Figure 2). Unsurprisingly, the majority of challenges apply to the free-range egg industry, where a larger range of factors increases the variability further, thus increasing the likelihood of compromised hen performance.
Variety of egg shell quality including calcification spots, pale eggs, elongated eggs, double yolkers and variable egg size. All eggs were obtained from the same flock during the first week of lay (18 weeks of age).

This article summarises common challenges seen in various housing systems and shares information that can help to overcome typical pitfalls. Furthermore, special emphasis is provided on new insights about the impact of free-range flock subpopulations on laying performance and egg quality.
CAGE SYSTEMS
Conventional Cages
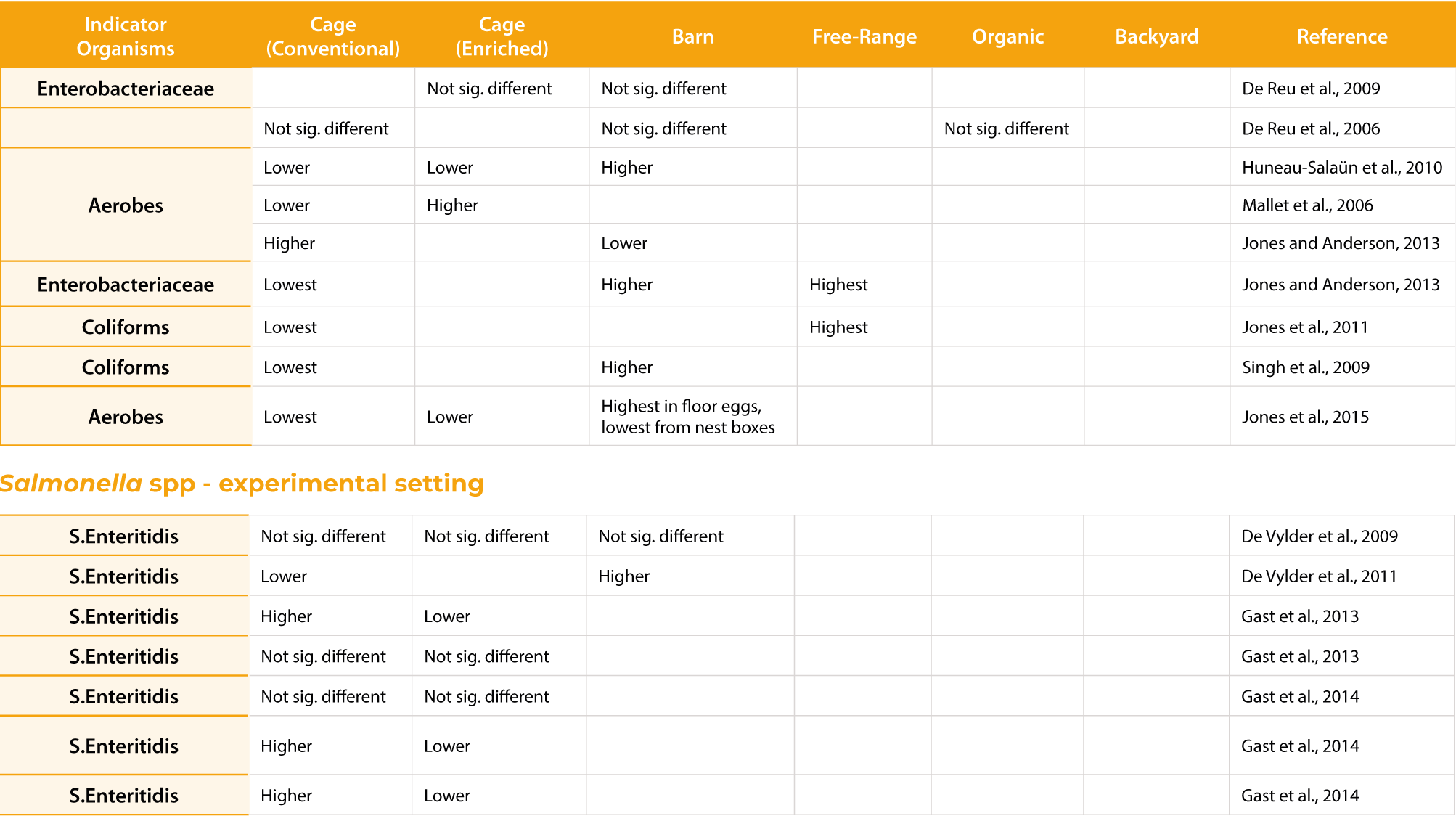
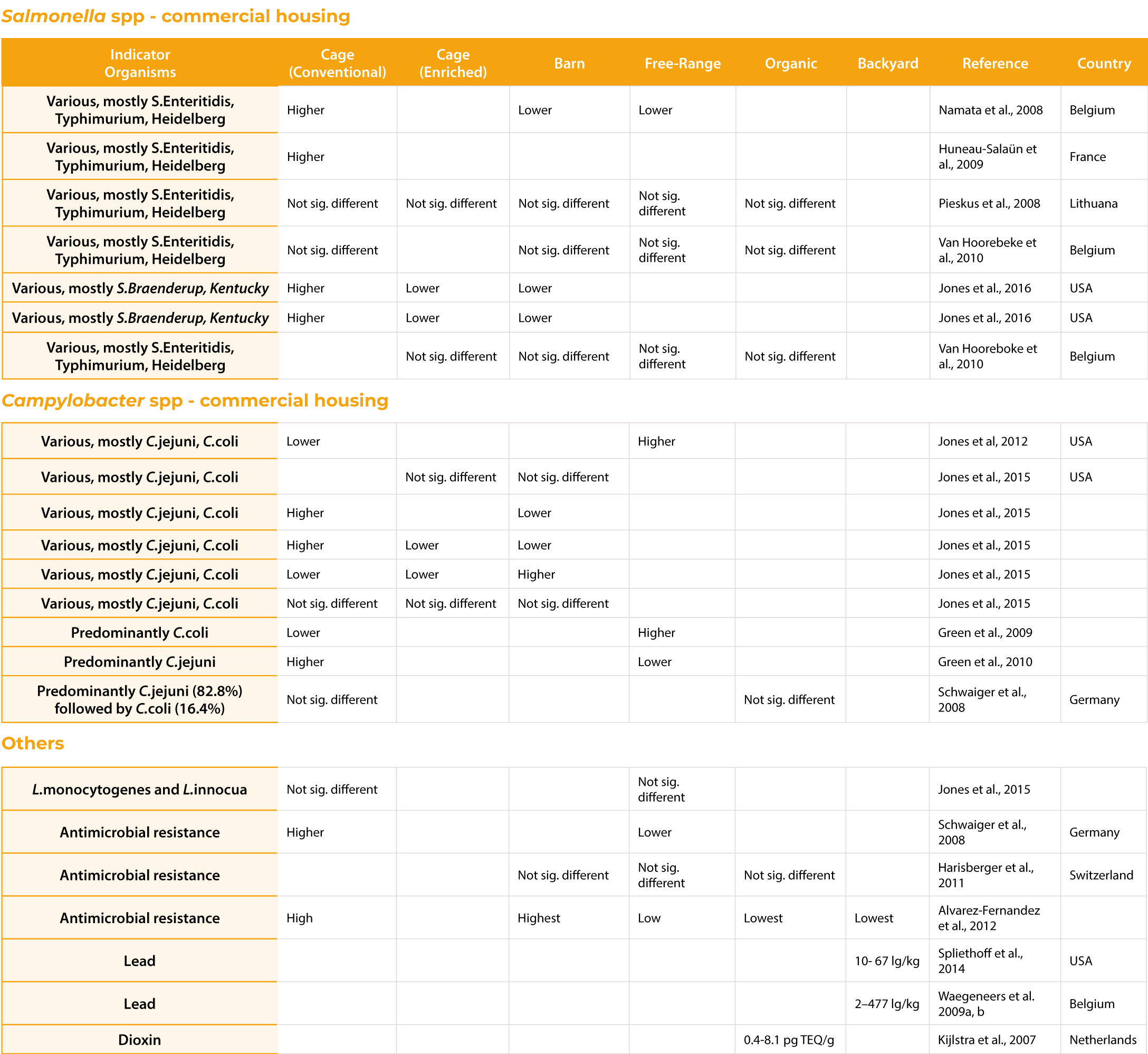
Hens housed in conventional cages demonstrated better egg production compared to furnished cages or noncages farming system when system or floor eggs were not managed, but showed equal results when hens were trained to use the nest boxes (Guesdon and Faure, 2004; Karcher et al., 2015; Wall and Tauson, 2002). The highest incidence of damaged eggs has been described in inadequately managed aviary and free-range systems and the lowest in cage systems (Abrahamsson and Tauson, 1995; Hughes et al., 1985). The design of the nest box can significantly improve the incidence of dirty eggs (Abrahamsson and Tauson, 1998) and can be a main reason for the equivocal results obtained in studies that compared conventional cages to furnished cages or aviaries (Abrahamsson et al., 1995; Abrahamsson and Tauson, 1998; Guesdon and Faure, 2004; Tauson et al., 1999). Similarly, equivocal results can be obtained regarding microbiota egg contamination, which is attributed mostly to dust exposure of the eggs associated with manure belt management, egg collection frequency, nestlocation, shed design, egg handling and various other factors (Carrique-Mas et al., 2009; Jones and Anderson, 2013; Tauson, 2002; Zhao et al., 2015; Figure 1). However, clear results have been obtained about the prevalence of the antimicrobial resistance associated with various bacterial contaminants (Alvarez-Fernandez et al., 2012; Harisberger et al., 2011; Schwaiger et al., 2008). While Alvarez-Fernandez et al., (2012) found 19.2% of all E. coli isolates to be susceptible to all antimicrobials tested, 80.8% of these samples were resistant to one (22.5%) or more (58.4%) antibiotics. Similar to the findings of Schwaiger et al., (2008) the highest prevalence of resistant strains was found in caged systems and was explained the highest stocking density and flock size, resulting in highest infection pressure which would require a more frequent use of antimicrobials.
Enriched Cages
Similar to conventional cage studies, research conducted on eggs produced in enriched cages has demonstrated equivocal results on egg quality and food safety (Jones et al., 2015; Jones et al., 2016; Mallet el at., 2006; Table 1; 2). The number of eggs laid outside the nest box and cracked eggs of enriched cages could be reduced by providing attractive nesting, resulting in comparable egg production between various housing or farming systems (Gast et al., 2013, Gast et al., 2014; Guesdon et al., 2006; Jones et al., 2015; Huneau-Salaün et al., 2010; de Reu et al., 2009).
The impact of the housing system on egg safety. All differences recorded were of statistical significance (p<0.05).
CAGE-FREE HOUSING SYSTEMS
Cage-free housing systems are designed to improve animal welfare by providing various features such as vertical space using perches and aviaries, but also horizontal space such as winter gardens (Rodenburg et al., 2005; Lay et al., 2011; Freire and Cowling, 2013). Common problems associated with cage-free systems include the production of overall fewer eggs, flocks not reaching the targeted breed standard, and a relatively high percentage of off-grade eggs (Aerni et al., 2005; Englmaierova et al., 2014; Golden et al., 2012; Singh et al., 2009; Tumova and Ebeid, 2003; Van Horne, 1996). A non-uniform use of resources contributes to the problem and can be observed in all cage-free (barn, free-range, organic) systems, but stress and flock size itself also increase the likelihood of misplaced or cracked eggs (De Haas et al., 2013; Sirovnik et al., 2018). Maintaining the egg shell quality is of paramount importance for non-cage systems, as the eggs produced to this standard need to be marketed and sold as whole eggs, covering the relatively higher housing costs.
Barn Housing
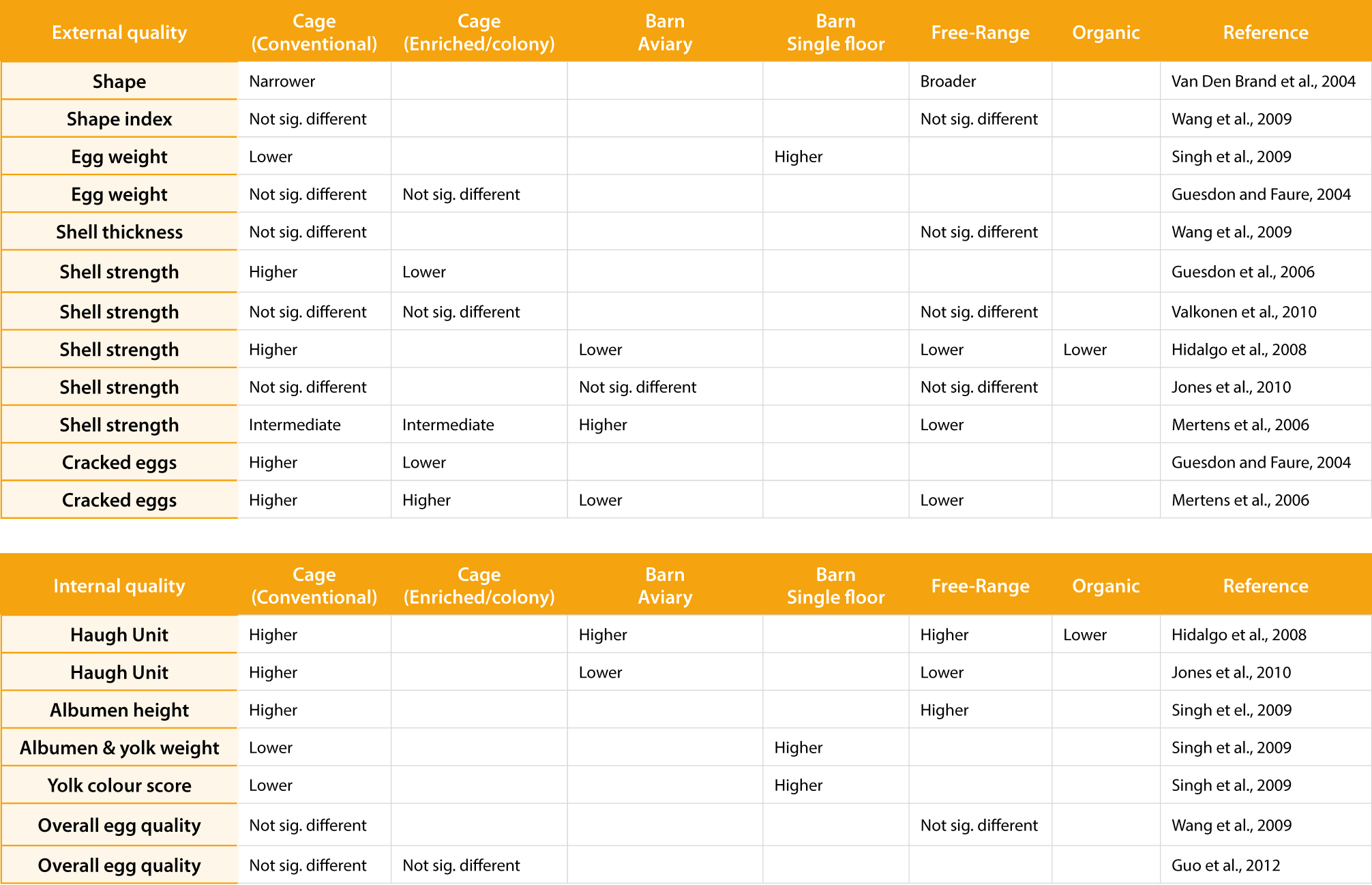
While barn systems do not dominate the egg market from a global perspective, it is the predominant housing system in some countries. For example, Colombia, Germany, Guatemala and The Netherlands house >60% of their hens in the barn and aviary system. Overall, the European Union (including the UK) houses 29% of their layers in the barn system, taking 119 million laying hens into account (https://ec.europa.eu/info/sites/info/files/foodfarmingfisheries/farming/documents/eggsdashboard_en.pdf). Ferrante et al. (2009) evaluated 4745 hens housed in commercial barns and compared their performance and egg quality with 2016 hens of the same strain housed in commercial organic/free-range systems. While the peak lay production of organic hens was superior compared to barn hens (94.5% vs 93.0%, respectively), the feed conversion ratio was worse (2.36 vs 2.20, respectively). However, significantly more eggs produced in the organic system were dirty while significantly more eggs laid in barn systems were cracked. Equivocal results have been obtained by other investigators indicating a later onset of lay or higher, equal or lower egg shell breaking strength compared to caged and/or free-range systems, as well as higher and/or lower Haugh Units (Hidalgo et al., 2008; Jones et al. 2010; Mertens et al; 2006; Singh et al., 2009; Van Den Brand et al., 2004; Valkonen et al.,2010). The fact that the interior of barn systems can vary greatly between and within countries (e.g. single floor slats, single floor housing with bedding material, aviary systems, aviary systems with winter garden) makes it challenging to compare study results to one another but also highlights that the cause of reduced egg quality is mostly multifactorial (Roberts and Chousalkar, 2009; Table 2)
The impact of the housing system on egg quality. All differences recorded were of statistical significance (p<0.05).
Free-Range Housing
Free range egg production is of increasing popularity world-wide, with certain countries such as Switzerland and the UK (57% of all hens housed free-range) leading the segment, followed by Australia (47%), Ireland (44%) and Austria (25%) (European Union overall 16%; Australian Eggs, 2019; Windhorst et al., 2013; Link)
It had been demonstrated numerous times that egg taste can be influenced by feed components such as fish oil or various spices (thyme, basil, garlic, fennel, peppermint, marjoram) if added at sufficient quantities to the layers’ diet (Tserveni-Gousi, 2001; Hammershøj and Steenfeld, 2012; Richter et al., 2002). These quantities may be up to 15g of spice/kg diet and are such not economically viable. Ranging hens usually denude their available area from plants in relatively short periods of time, eating up to 60 g pasture/hen/day. This leads to negligible pasture intake over the duration of an entire laying period unless being provided by continuously new range areas due to rotational fencing or mobile sheds that frequently move to new grazing areas (Singh and Cowieson, 2013; Ruhnke et al., 2015). The practice of mobile sheds is especially used by grain farmers, who run their flocks on harvested fields, expecting a fertiliser effect from the chicken manure as well as a cleaned-up paddock where the hens eat loose grains remaining in the stubble. Foraging in general and on lucerne in particular increases yolk colouring, adding betacarotenes, canthaxanthins’ lutein and xanthophylls such as violaxanthin to the diet which is deposited in the egg yolk (Ahammed at al., 2014;
Ferrante et al., 2009; Pavlovski et al, 2001; Golden et al, 2012). In contrast, naked oats fed at 800g/kg decreased yolk colour in addition to yolk flavour intensity (Cave et al., 1992). Other forage material may include silages or kale with varying effects depending on the quantity consumed (Hammershøj and Steenfeld, 2012). However, in most flocks where hens are housed for commercial production, the intake of additional forage material diet is negligible and therefore sensory properties of eggs are usually not affected by the production or farming system (Mizumoto et al. 2008).
Soil intake during range use can also significantly impact egg quality, challenging food safety. Of special concern are fat soluble heavy metals such as dioxin or lead, which will be covered in the section “organic housing systems”; further below, as organic hens seem to be significantly more often affected, most likely due
to smaller flock sizes which is known to correlate positive with range use.
It has previously been proposed that, due to the greater physical activity of free-range hens, increased bone strength may occur, leading to a better calcium deposition which would then be available for egg shell production later in life (Miao et al., 2005). While early range access has been linked to increased hen body
weight and egg production, an impact on bone quality could not be seen. This was most likely due to the provision of an aviary system in the shed during rearing and lay, which encourages hens to jump vertically which would have diminished any potential beneficial effect of horizontal range use on bone quality (Kolakshyapati et al., 2019a; Sibanda et al., 2018; Sibanda et al., 2020a). The diversity of non-uniform hen behaviours and their impact on the hen’s physiology and health status, and subsequently egg performance, is even greater in free-range farming systems compared to barn housed chickens. Range use allows for UVlight exposure in addition to the photo stimulation provided by the artificial light provided in the hen house and therefore further increases the variation of ovulation
rate and calcium deposition (Coletta et al., 2012; England and Ruhnke, 2020; Fanatico, 2006; Kolakshyapati et al., 2020; Lewis and Gous, 2009; Siopes and Wilson; 1980). The fact that some hens rarely or never leave the shed while others range daily, reduced body weight uniformity and inconsistent egg production may occur (Gebhardt-Henrich et al. 2014a,b; Gilani et al., 2014; Sibanda et al., 2019).
Investigating the effects of range use allows egg producers an in-depth understanding of their flock and subsequently improved decision making resulting in increased egg production (Gocsik et al., 2014; Wolc et al., 2011). Icken et al. (2008) investigated winter garden use and laying performance and detected a low negative correlation between laying performance and the frequency as well as the duration of winter garden use (). The authors investigated hen movement and
nest box usage daily for one laying cycle. They suggested that hens that often accessed the winter garden area may have not returned to the nest boxes for laying. Unfortunately, the authors did not collect floor eggs and as such were not able to correlate those eggs with a hen’s movement patterns. A similar approach was used by Sibanda et al (2020b), who tracked individual hens to determine the differences in laying rate and egg quality of flock sub-populations with different range use during early age (18-21 weeks of age). While Icken et al. (2008) investigated 272 Lohmann Silver hens in experimental facilities, Sibanda et al investigated 15625
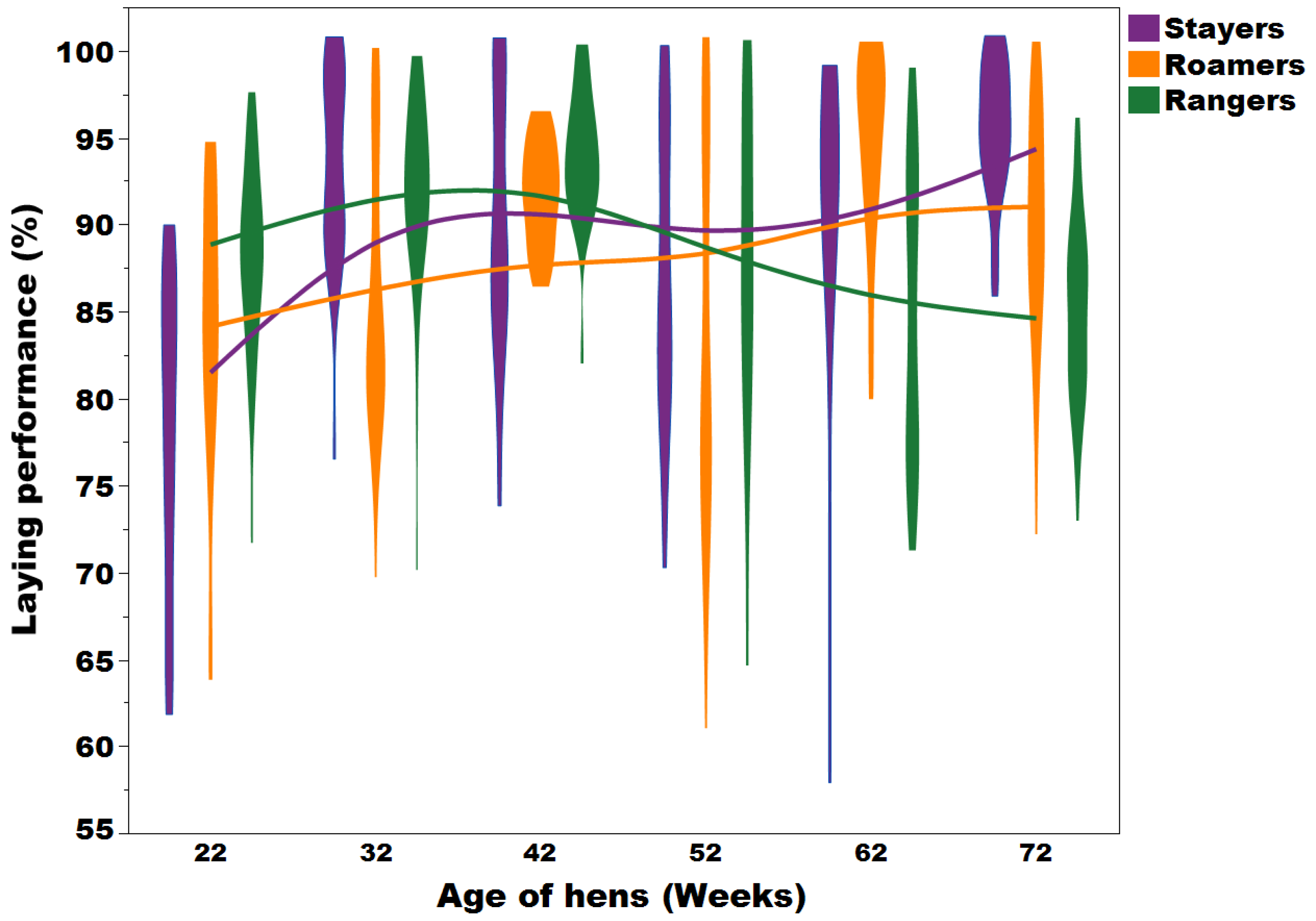
Lohmann Brown hens being part of 5 commercial flocks. A limitation of Sibanda et al was that the hens were monitored for their range use from 18-21 weeks of age and based on this range use grouped into “stayers” (hens that spent most of their time in the shed), “roamers” (hens that accessed the range infrequently) and
“rangers” (hens that spent most of their time on the range). All of these groups continued to have access to the commercial facilities including an aviary system and, as such, eggs from these individual groups could not be collected daily (and therefore hen/ house production not be determined for the entire laying period), but were collected in 10-weekly intervals. Significant differences were noted for hen-day production at different hen ages: At 22 weeks of age, rangers and stayers had a laying performance of 88.0 % and 78.2%, respectively, but at 72 weeks of age the peaks had shifted and rangers laid about 10% fewer eggs compared to stayers (laying performance of rangers and stayers was 85.1% and 95.5%, respectively (all P <0.05; Figure 3). However, there was no overall effect of early range use on laying performance. These results lead to the suspicion that the previously reported reduced laying performance of free-range flocks may be attributed to a lower
performance of flock sub-populations rather than the housing system as such and supports findings where free-range flocks perform equally or exceed the performance of caged hens. In addition, range use did not significantly affect the egg quality (Figure 4, 5, 6, and 7). The fact that range use had no negative impact on egg quality suggests no reasons to be concerned about range use from this point of view. However, the impact of range use on hen heath and mortalities and subsequently
total egg outputs needs further investigation.
A violin plot with an overlay of smooth spline (lambda = 3.5) representing the distribution and the laying performance trend of stayers (purple), roamers (orange) and rangers (green) at 22, 32, 42, 52, 62, and 72 weeks of age per day in commercial free-range laying hens. The time-age interaction can be seen at 52 weeks of age (Figure adapted from Sibanda et al., 2020b). This figure has been published in Feedmgazine/Kraftfutter, Dec. 2023.
A violin plot with an overlay of smooth spline (lambda = 3.5) representing the trends and distribution between the egg weight and albumen height of stayers (purple), roamers (orange), and rangers (green) at 22, 32, 42, 52, 62 and 72 weeks of age (Figure adapted from Sibanda et al., 2020b).
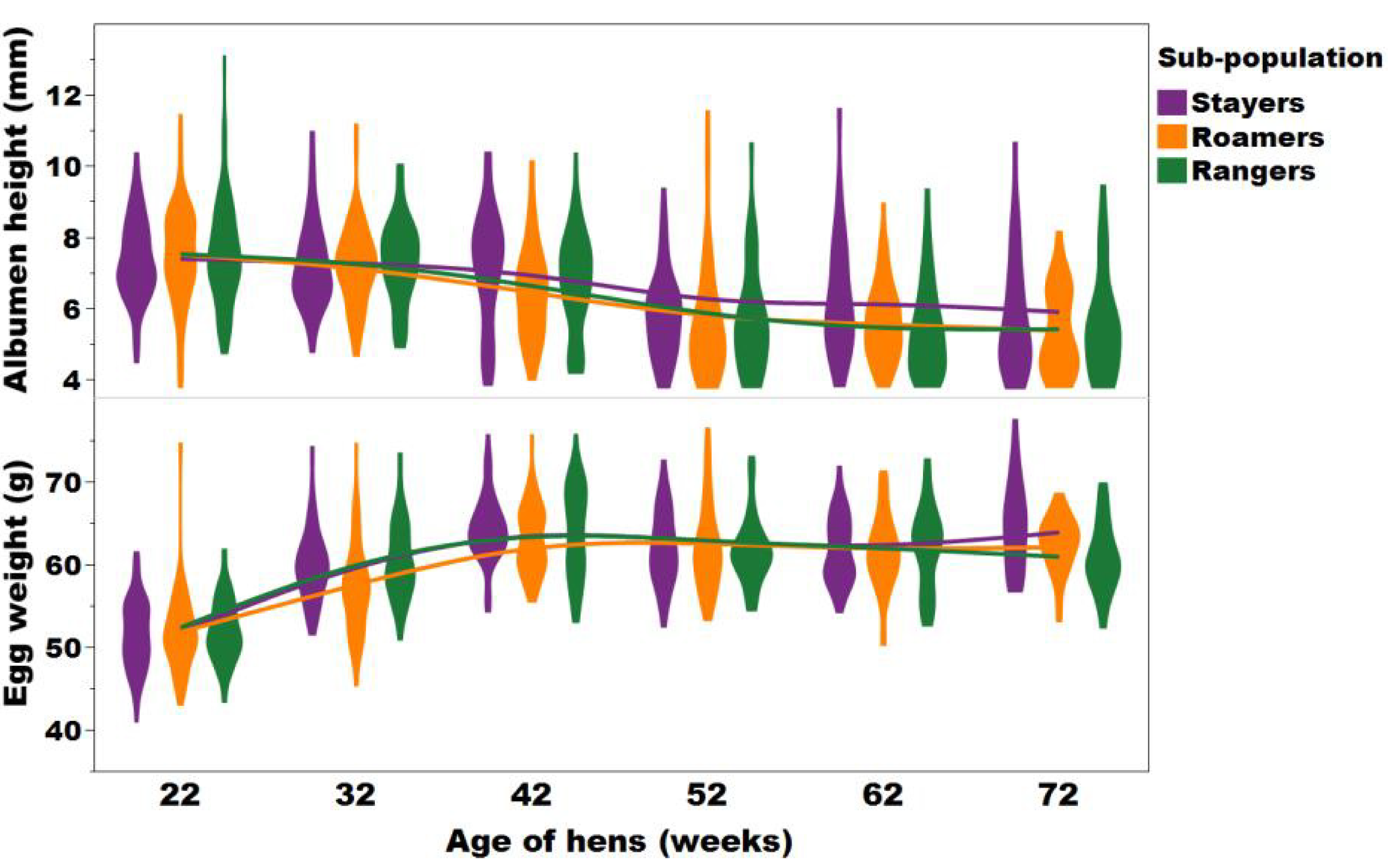
A violin plot with an overlay of smooth spline (lambda = 3.5) representing the trends and distribution between the egg yolk colour and Haugh unit of the stayers (purple), roamers (orange), and rangers (green) at 22, 32, 42, 52, 62, and 72 weeks of age (Figure adapted from Sibanda et al., 2020b).
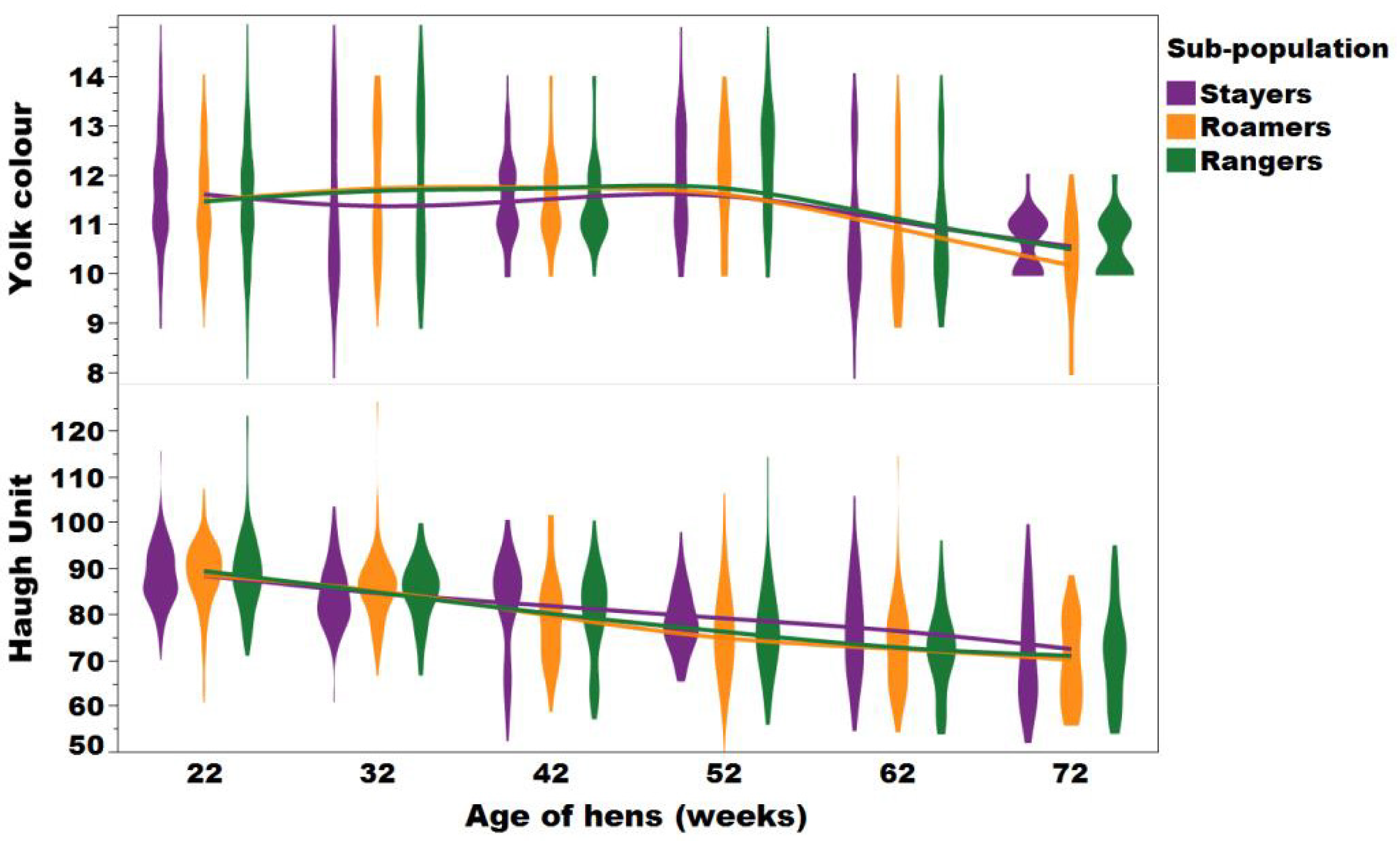
A violin plot with an overlay of smooth spline (lambda = 3.5 representing the trends, interaction and distribution between the eggshell breaking strength of the stayers (purple), roamers (orange) and rangers (green) at 22, 32, 42, 52, 62, and 72 weeks of age (Figure adapted from Sibanda et al., 2020b).
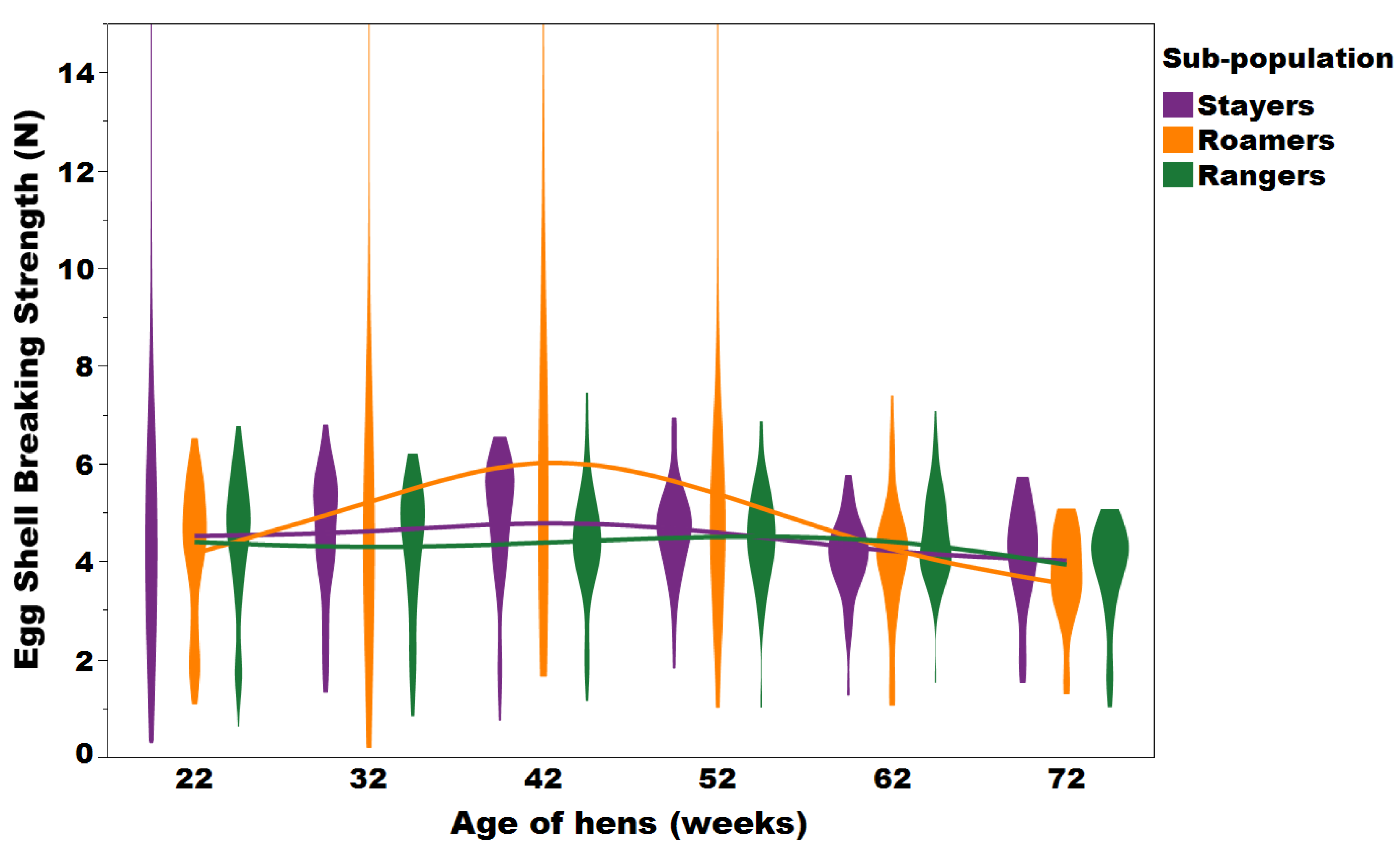
A violin plot with an overlay of smooth spline (lambda = 3.5) representing the trends of system, floor and waste eggs collected from the various flock sub-populations at 22, 32, 42, 52, 62, and 72 weeks of age (Figure adapted from Sibanda et al., 2020b). This figure has been published in Feedmagazine/Kraftfutter, Dec. 2023.
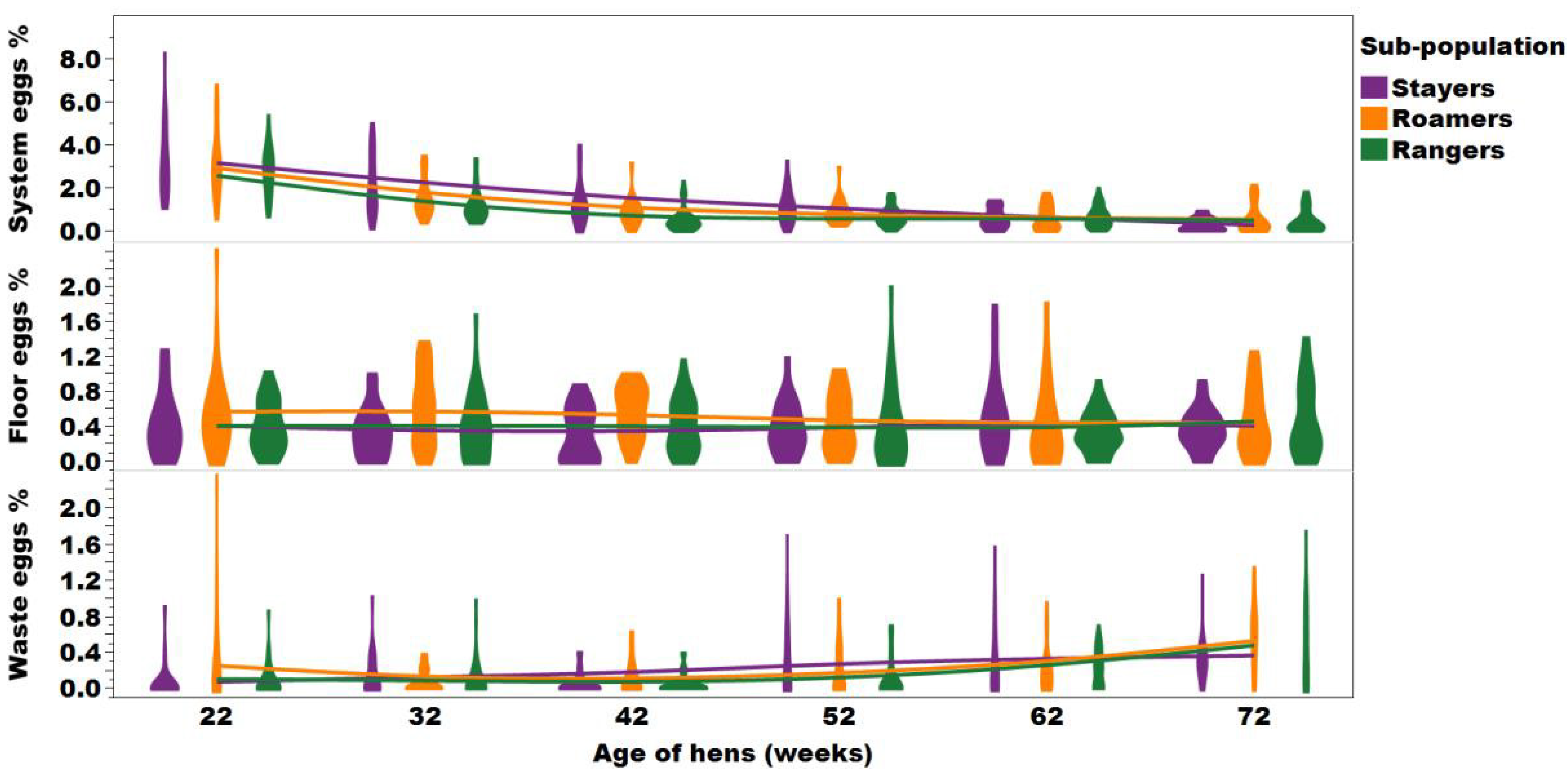
Barnett et al., (1992) provided evidence that fear of humans was a factor that was negatively correlated with peak hen day production, coming into effect when birds were exposed to unfamiliar environments. With stayers being more fearful than rangers, the higher blood cortisol levels in combination with reduced UV light exposure in stayers may explain the lower egg performance and it would be interesting to investigate further effects on egg shell colour, for example, as shell colour has been found to be paler in free-range flocks compared to barn or caged eggs (Campbell et al., 2016; Hartcher et al., 2016; Kolakshyapati et al., 2019b;
Mahboub et al., 2004; Lewis and Gous, 2009; Siopes and Wilson; 1980; Roberts and Chousalkar, 2009). The increased fearfulness of stayers may have also been a reason for the increased presence of system eggs (e.g. eggs laid in areas of the aviary system that were not nest boxes; Figure 7). System and floor eggs increase not only the labor associated with egg collection, but affect egg shell hygiene, preventing these eggs from being sold as whole in some countries such as member states of the European Union and therefore presenting a significant financial loss to the producer (Van den Brand et al., 2004; Michel and Huonnic, 2003; Jones et al; 2015; Villanueva et al., 2017).
Range use did not impact egg shell breaking strength and, at 72 weeks of age, the breaking strength remained comparable to that at the beginning of lay, indicating the ability to house hens beyond the commonly practiced 72 weeks of age (Bain et al., 2016).
Soil ingestion from the range can result in the accumulation of elevated dioxin-like compounds where free-range eggs have higher and polychlorinated biphenyl levels than conventional cage eggs (Schoeters and Hoogenboom, 2006).
Organic Housing
Organic egg production is the least sustainable housing or farming system, taking ammonia emissions from manure, reduced number of eggs produced per hen per year and higher feed conversion ratio into account when conducting life cycle assessments (Aarnik et al., 2006; Dekker et al., 2009; Nijdam et al., 2012; Williams et al., 2006). While the increased use of food and land resources have a major impact on the carbon footprint of the industry, the sector is rapidly growing in many countries and eggs are usually sold at a premium, allowing this sector to have the highest profit margin. Therefore, the organic sector has a relatively high potential to increase its sustainability and efficiency by producing more eggs/hen, resulting at the same time in the highest financial reward when being able to sell more egg outputs at a relatively high price (Australian Eggs, 2019). The definition and underlying legislation vary largely world-wide, but common challenges affecting the quality of organic eggs include unintentional impacts of the environment such as contaminated soil ingestion. Vincevica-Gaile et al. (2013) investigated various trace elements in eggs obtained from different housing or farming systems and eggs derived from organic farms had the highest concentrations of most elements. While this is not of general concern, the unwanted exposure to contaminants becomes critical when affecting food safety. Kiljstra et al (2007) investigated 34 organic farms in Europe of which 9 (nearly 10% of the investigated farms) exceeded the legal limit of 3 pg of toxic equivalence/g of egg fat. With dioxin being ubiquitously present in the soil of many industrialized countries, its presence in eggs seems to be positively correlated to decreasing flock size and as such affecting organic flocks which are commonly smaller more often than larger free-range flocks (Kiljstra et al., 2007). Flock size significantly affects the percentage of hens that access the range, increasing the exposure time of the ranging hens to the contaminated environment and the amount of soil uptake (Harnly et al., 2000; Hegelund et al., 2005). Thus, it is not surprising that organic farms worldwide have been affected by dioxin-like compounds such as polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF) and polychlorinated biphenyls (Harnly et al., 2000). Similarly, pesticides and heavy metal contamination of the environment such as dichlorodiphenyltrichloroethane (DDT) or lead can be frequently elevated. Eggs obtained from backyard flocks in Belgium had 2-6 times higher concentrations of lead, mercury, cobalt and thallium than those obtained from commercial farms (Spliethoff et al., 2014; Van Overmeire et al., 2006; Waegeneers et al. 2009a, b). Despite the fact that the interaction of hens with the living environment such as rodents, insects, wild birds and mammals is theoretically higher in free-range and organic housing or farming systems, the incidence of biological contamination with food borne pathogens such as Salmonella enteritidis, Listeria monocytogenes or various Campylobacter species is not necessarily higher than in caged farming systems (Schwaiger et al., 2008; Van Hoorebeke et al., 2010; Wales et al., 2007). As mentioned above, resistance rates of bacterial isolates from cage farming systems have higher values than those from organic ones (Alvarez-Fernandez et al., 2012; Schwaiger et al., 2008). These results show that biosecurity challenges in free-range and organic systems can be effectively addressed, reducing inter-flock and organic material transmission to insignificant levels.
Conclusion and implications
Housing or farming system have no impact on egg shape but inconsistent information has been obtained on egg weight, egg shell breaking strength, shell thickness, cracked eggs and dirty eggs. An impact on shell colour could be observed where free-range hens had lighter shell colour compared to barn and caged hens. The production or farming system may affect food safety and egg quality especially due to ingestion of dioxin and lead contaminated soil, but does not necessarily impact the Salmonella prevalence as all farming systems are able to be managed to produce eggs of superior external and internal quality. Nest box management is of highest impact as its design and management determine the hen’s access time as well as dust exposure. While egg production differs with range usage, egg quality between ranging and non-ranging hens is of minor relevance. Awareness of the typical pitfalls associated with the different production or farming systems and their impact on egg quality allows for prevention and preparedness, resulting in the reliable outcome of immaculate and safe eggs with minimal losses and hence improved sustainability of the industry.
Acknowledgement
Data of flock sub-populations and egg quality were extracted based on the publication “Sibanda TZ, Kolakshyapati M, Welch M, Schneider D, Boshoff J, Ruhnke I (2020b) Managing free-range laying hens – Part A: Frequent and non-frequent range users differ in laying performance but not egg quality Animals 10:991 https://doi.org/10.3390/ani1006099 “. This research was funded by Australian Eggs and the Poultry CRC, established and supported under the Australian Government’s Cooperative Research Centres Program. We thank the commercial farm involved for outstanding support in all areas including the use of infrastructure, excellent communication and teamwork.
LOHMANN Information is a recognized, scientific publication in English language, focused on life science, human nutrition, animal and poultry breeding, production, nutrition, health and welfare. LOHMANN BREEDERS has a history of supporting this independent publication in its distribution. The content is the responsibility of the editor Prof Werner Bessei (Professor em. Farm Animal Ethology and Small Animal Science) and is coordinated with the authors.
References
Aarnink, A.J., Hol, J.M.G., Beurskens, A.G. (2006) Ammonia emission and nutrient load in outdoor runs of laying hens. Njas-Wageningen Journal of Life Sciences, 54(2): 223-234.Abrahamsson, P., Tauson, R. (1998) Performance and egg quality of laying hens in an aviary system. J. Appl. Poult. Res., 7(3): 225-232.
Abrahamsson, P., Tauson, R., Appleby, M.C. (1995) Performance of four hybrids of laying hens in modified and conventional cages. Acta Agr. Scand. A-Animal Sciences, 45(4), 286-296.
Aerni, V., Rinkhof, M.W.G., Wechsler, B., Oester, H., Frohlich, E. (2005) Productivity and mortality of laying hens in aviaries: a systematic review. World Poultry Sci. J. 61: 130-142.
Ahammed, M., Chae, B.J., Lohakare, J., Keohavong, B., Lee, M.H., Lee, S.J., Kim, D.M., Lee, J.Y., Ohh S.J. (2014) Comparison of Aviary, Barn and Conventional Cage Raising of Chickens on Laying Performance and Egg Quality. Asian-Australas J. Anim Sci. 27: 1196-1203.
Alvarez-Fernandez, E., Dominguez-Rodriguez, J., Capita, R., Alonso-Calleja, C. (2012) Influence of housing systems on microbial load and antimicrobial resistance patterns of Escherichia coli isolates from eggs produced for human consumption. J. Food Prot. 75(5): 847-853.
Australian eggs (2019) Annual report, Australian eggs.
Bain, M.M., Nys Y., Dunn I.C. (2016) Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 57(3): 330-338, DOI: 10.1080/00071668.2016.1161727.
Barnett, J.L., Hemsworth, P.H., Newman, E.A. (1992) Fear of humans and its relationships with productivity in laying hens at commercial farms. Br. Poult. Sci. 33(4): 699-710.
Bestman, M.W.P., Wagenaar, J.P. (2003) Farm level factors associated with feather pecking in organic laying hens. Livest. Prod. Sci. 76: 559–563.
Campbell, D.L., Hinch, G.N., Downing, J.A., Lee, C. Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. (2016) Appl. Anim. Behav. Sci. 185:73-77.
Cave, N.A., Farnworth, E.E., Poste, L.M., Butler, G., Burrows, V.D. (1992) Effect of dietary level of naked oats (Avena nuda) on internal and sensory quality of eggs and on yolk lipid composition. Can. J. Animal Sci. 72(1): 147-153.
Carrique-Mas, J.J., Breslin, M., Snow, L., McLaren, I., Sayers, A.R., Davies, R.H. (2009) Persistence and clearance of different Salmonella serovars in buildings housing laying hens. Epidemiol. Infect. 137(6): 837-846.
Coletta, L.D., Pereira, A.L., Coelho, A.A.D., Savino, V.J.M., Menten, J.F.M., Correr, E., Franca, L.C., Martinelli, L.A. (2012) Barn vs. free-range chickens: Differences in their diets determined by stable isotopes. Food Chem. 131: 155-160.
De Haas, E.N., Kemp, B., Bolhuis, J.E., Groothuis, T., Rodenburg, T.B. (2013) Fear, stress, and feather pecking in commercial white and brown laying hen parent-stock flocks and their relationships with production parameters. Poultry Sci. 92(9): 2259-2269.
De Reu, K., Rodenburg, T.B., Grijspeerdt K., Messens W., Heyndrickx, M., Tuyttens F.A.M., Sonck, B., Zoons, J., Herman, L. (2009) Bacteriological contamination, dirt, and cracks of eggshells in furnished cages and noncage systems for laying hens: An international on-farm comparison. Poultry Sci 88(11): 2442-2448.
De Reu, K., Grijspeerdt, K., Heyndrickx, M., Uyttendaele, M., Debevere, J., Herman, L. (2006) Bacterial shell contamination in the egg collection chains of different housing systems for laying hens. Br. Poult. Sci. 47(2): 163-172.
De Reu, K., Grijspeerdt, K., Heyndrickx, M., Zoons, J., De Baere, K., Uyttendaele, M., Debevere, J. Herman, L. (2005) Bacterial eggshell contamination in conventional cages, furnished cages and aviary housing systems for laying hens. Br. Poult. Sci. 46(2): 149-155.
De Vylder, J., Van Hoorebeke, S., Ducatelle, R., Pasmans, F., Haesebrouck, F., Dewulf, J., Van Immerseel, F. (2009) Effect of the housing system on shedding and colonization of gut and internal organs of laying hens with Salmonella Enteritidis. Poultry Sci. 88(12): 2491-2495.
De Vylder, J., Dewulf, J., Van Hoorebeke, S., Pasmans, F., Haesebrouck, F., Ducatelle, R., Van Immerseel, F. (2011) Horizontal transmission of Salmonella Enteritidis in groups of experimentally infected laying hens housed in different housing systems. Poultry Sci. 90(7): 1391-1396.
Dekker, S. E. M., De Boer, I. J. M., Aarnink, A. J. A., Koerkamp, P. G. (2009) Enviromental hotspot identification of organic egg production. In Proceedings of the 6th International Conference on LCA in the Agrifood Sector, Zurich, Switzerland, November 12-14, 2008; 381-389.
England, A., Ruhnke, I., (2020) The influence of light of different wavelengths on laying hen production and egg quality. World Poultry Sci. J. – accepted
Englmaierova, M., Tumova, E., Charvatova, V., Skrivan, M. (2014) Effects of laying hens housing system on laying performance, egg quality characteristics, and egg microbial contamination. Czech J. Anim. Sci. 59: 345-352.
Eurostat (2011) Food: from farm to fork statistics, Eurostat Pocketbook, 2011 edition. Available at: http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-32-11-743/EN/KS-32-11-743-EN.PDF
Fanatico (2006) Alternative poultry production systems and outdoor access. A publication of ATTRANational Sustainable Agriculture Information service, 1-800-346-9140. www.attra.ncat.org
Ferrante, V., Lolli, S., Vezzoli, G., Cavalchini, L. G. (2009) Effects of two different rearing systems (organic and barn) on production performance, animal welfare traits and egg quality characteristics in laying hens. Ital. J. Animal Sci. 8(2): 165-174.
Freire, R., Cowling, A. (2013) The welfare of laying hens in conventional cages and alternative systems: first steps towards a quantitative comparison. Anim. Welf. 22:57-65.
Gast, R.K., Guraya, R., Jones, D.R., Anderson, K.E. (2014) Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens housed in conventional or enriched cages. Poultry Sci. 93(3): 728-733.
Gebhardt-Henrich, S.G., Toscano, M.J., Frohlich, E.K.F. (2014a) Use of outdoor ranges by laying hens in different sized flocks. Appl. Anim. Behav. Sci. 155: 74-81.
Gebhardt-Henrich, S., Fröhlich, E., Burose, F., Fleurent, J., Gantner, M., Zähner, M. (2014b) Individual tracking of laying hens with an RFID-System. Landtechnik 69: 301-306.
Gilani, A.M., Knowles, T.G., Nicol, C.J. (2014) Factors affecting ranging behaviour in young and adult laying hens. Br. Poult. Sci. 55(2): 127-135.
Gole, V.C., Chousalkar, K.K., Roberts, J.R. (2013) Survey of Enterobacteriaceae contamination of table eggs collected from layer flocks in Australia. Int. J. Food Microbiol. 164(2-3): 161-165.
Golden, J.B., Arbona, D.V., Anderson, K.E. (2012) A comparative examination of rearing parameters and layer production performance for brown egg-type pullets grown for either free-range or cage production. J. Appl. Poult. Res. 21(1): 95-102.
Gocsik, E., Saatkamp, H.W., De Lauwere, C.C., Lansink, A.O. (2014) A conceptual approach for a quantitative economic analysis of farmers’ decision-making regarding animal welfare. J. Agr. Envir. Ethics, 27(2): 287-308.
Green, A.R., Wesley, I., Trampel, D.W., Xin, H. (2009) Air quality and bird health status in three types of commercial egg layer houses. J. Appl. Poult. Res. 18(3): 605-621.
Guesdon, V., Faure, J. (2004) Laying performance and egg quality in hens kept in standard or furnished cages. Animal Res. EDP Sciences, 53(1): 45-57.
Guesdon, V., Ahmed, A.M.H., Mallet, S., Faure, J.M., Nys, Y. (2006) Effects of beak trimming and cage design on laying hen performance and egg quality. Br. Poult. Sci. 47(1): 1-12.
Hammershøj, M., Steenfeldt, S. (2012) The effects of kale (Brassica oleracea ssp. acephala), basil (Ocimum basilicum) and thyme (Thymus vulgaris) as forage material in organic egg production on egg quality. Br. Poult. Sci. 53(2): 245-256.
Harisberger, M., Gobeli, S., Hoop, R., Dewulf, J., Perreten, V., Regula, G. (2011) Antimicrobial resistance in Swiss laying hens, prevalence and risk factors. Zoonoses Publ. Hlth 58(6): 377-387.
Harnly, M.E., Petreas, M.X., Flattery, J., Goldman, L.R. (2000) Polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran contamination in soil and home-produced chicken eggs near pentachlorophenol sources. Envir. Sci. Technol. 34(7): 1143-1149.
Hartcher, K., Hickey, K., Hemsworth, P., Cronin, G., Wilkinson, S., Singh, M. (2016) Relationships between range access as monitored by radio frequency identification technology, fearfulness, and plumage damage in free-range laying hens. Animal 10: 847-853.
Hegelund, L., Sørensen, J.T., Kjaer, J.B., Kristensen, I.S. (2005) Use of the range area in organic egg production systems: effect of climatic factors, flock size, age and artificial cover. Br. Poult. Sci. 46(1): 1-8.
Hidalgo, A., Rossi, M., Clerici, F., Ratti, S. (2008) A market study on the quality characteristics of eggs from different housing systems. Food Chem. 106(3): 1031-1038.
Huneau-Salaün, A., Chemaly, M., Lalande, F., Petetin, I., Rouxel, S., Michel, V., Fravalo, P., Rose, N. (2009) Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 89(1-2): 51-58.
Huneau-Salaün, A., Michel, V., Huonnic, D., Balaine, L., Le Bouquin, S. (2010) Factors influencing bacterial eggshell contamination in conventional cages, furnished cages and free-range systems for laying hens under commercial conditions. Br. Poult. Sci. 51(2): 163-169.
Holt, P.S., Davies, R.H., Dewulf, J., Gast, R.K., Huwe, J.K., Jones, D.R., Waltman, D. and Willian, K.R. (2011) The impact of different housing systems on egg safety and quality. Poultry Sci. 90(1): 251-262.
Icken, W., Cavero, D., Schmutz, M., Thurner, S., Wendl, G., Preisinger, R. (2008) Analysis of the free range behaviour of laying hens and the genetic and phenotypic relationships with laying performance. Br. Poult. Sci. 49(5): 533-541.
Jones, D.R., Anderson, K.E. (2013) Housing system and laying hen strain impacts on egg microbiology. Poultry Sci. 92(8): 2221-2225.
Jones, D.R., Musgrove, M.T., Anderson, K.E., Thesmar, H.S. (2010) Physical quality and composition of retail shell eggs. Poultry Sci. 89(3): 582-587.
Jones, D.R., Anderson, K.E., Guard, J.Y. (2012) Prevalence of coliforms, Salmonella, Listeria, and Campylobacter associated with eggs and the environment of conventional cage and free-range egg production. Poultry Sci. 91(5): 1195-1202.
Jones, D.R., Anderson, K.E., Musgrove, M.T. (2011) Comparison of environmental and egg microbiology associated with conventional and free-range laying hen management. Poultry Sci. 90(9): 2063-2068.
Jones, D.R., Cox, N.A., Guard, J., Fedorka-Cray, P.J., Buhr, R.J., Gast, R.K., Abdo, Z., Rigsby, L.L., Plumblee, J.R., Karcher, D.M. and Robison, C.I. (2015) Microbiological impact of three commercial laying hen housing systems. Poultry Sci. 94(3): 544-551.
Karcher, D.M., Jones, D.R., Abdo, Z., Zhao, Y., Shepherd, T.A., Xin, H. (2015) Impact of commercial housing systems and nutrient and energy intake on laying hen performance and egg quality parameters. Poultry Sci. 94(3): 485-501.
Kijlstra, A., Traag, W.A., Hoogenboom, L.A.P. (2007) Effect of flock size on dioxin levels in eggs from chickens kept outside. Poultry Sci. 86(9): 2042-2048.
Kolakshyapati, M., Flavel, R.J., Sibanda, T.Z., Schneider, D., Welch, M.C., Ruhnke, I. (2019a). Various bone parameters are positively correlated with hen body weight while range access has no beneficial effect on tibia health of free-range layers. Poultry Sci. 98(12): 6241-6250.
Kolakshyapati, M.; Taylor, P.S.; Sibanda, T.Z.; Ruhnke, I. (2019b) Association of fearfulness at the end of lay with range visits during 18-22 weeks of age in commercial laying hens. In Proceedings of the Congress of the International Society for Applied Ethology, Bergen, Norway, 5th-9th August, 2019.
Kolakshyapati, M., Wu, S.-B., Sibanda, T.Z., Ramirez-Cuevas, S., Ruhnke, I. (2020) Body weight and range usage affect net energy utilisation in commercial free-range laying hens when evaluated in net energy chambers. Animal Nutrition. https://doi.org/10.1016/j.aninu.2019.12.003
Lay, D.C., Fulton, R.M., Hester, P.Y., Karcher, D.M., Kjaer, J.B., Mench, J.A., Mullens, B.A., Newberry, R.C., Nicol, C.J., O’Sullivan, N.P., Porter, R.E. (2011) Hen welfare in different housing systems. Poultry Sci. 90:278-294.
Lambton, S.L., Knowles, T.G., Yorke, C., Nicol, C.J. (2010) The risk factors affecting the development of gentle and severe feather pecking in loose housed laying hens. Appl. Anim. Behav. Sci. 123: 32–42.
Lewis, P.D., Gous, R.M. (2009) Responses of poultry to ultraviolet radiation. Worlds Poultry Sci. J. 65:499–510.
Lordelo, M., Fernandes, E., Bessa, R.J.B., Alves, S.P. (2016) Quality of eggs from different laying hen production systems, from indigenous breeds and specialty eggs. Poultry Sci. 96: 1485-1491.
Mahboub, H.D.H., Müller, J., Von Borell, E. (2004) Outdoor use, tonic immobility, heterophil/lymphocyte ratio and feather condition in free-range laying hens of different genotype. Br. Poult. Sci. 45(6): 738-744.
Mallet, S., Guesdon, V., Ahmed, A.M.H., Nys, Y. (2006) Comparison of eggshell hygiene in two housing systems: Standard and furnished cages. Br. Poult. Sci. 47(1): 30-35.
Mertens, K., Bamelis, F., Kemps, B., Kamers, B., Verhoelst, E., De Ketelaere, B., Bain, M., Decuypere, E. and De Baerdemaeker, J. (2006) Monitoring of eggshell breakage and eggshell strength in different production chains of consumption eggs. Poultry Sci. 85(9): 1670-1677.
Miao, Z.H., Glatz, P.C., Ru, Y.J. (2005) Free-range poultry production-A review. Asian-Australasian J. of Anim. Sci. 18(1): 113-132.
Michel, V., Huonnic, D. (2003) A comparison of welfare, health and production performance of laying hens reared in cages or aviaries. Br. Poult. Sci. 44(5): 775-776. DOI: 10.1080/00071660410001666691.
Mizumoto, E.M., Canniatti-Brazaca, S.G., Vasques Farinazzi Machado, F.M. (2008). Chemical and sensorial evaluation of eggs obtained by different production systems. Ciencia Tecnol. Aliment. 28(1):60-65.
Namata, H., Méroc, E., Aerts, M., Faes, C., Abrahantes, J. C., Imberechts, H., Mintiens, K. (2008) Salmonella in Belgian laying hens: an identification of risk factors. Prev. Vet. Med. 83(3-4): 323-336.
Nijdam, D., Rood, T., Westhoek, H. (2012): The price of protein: Review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Pol. 37(6): 760-770.
Parrott, P.A.W. (2004) Hen welfare: the consumers’ perspective. Welfare of the Laying Hen. In: Poultry Science Symposium Series 27: 11-22. CABI International, Oxford, UK.
Palvlovski Z., Hopic S., Lukic M. (2001) Housing systems for layers and egg quality. Biotech. Anim. Husb. 17:197–201.
Pieskus, J., Kazeniauskas, E., Butrimaite-Ambrozeviciene, C., Stanevicius, Z., Mauricas, M. (2008) Salmonella incidence in broiler and laying hens with the different housing systems. J. Poult. Sci. 45(3):227-231.
Richter, T., Braun, P., Fehlhaber, K. (2002) Influence of spiced feed additives on taste of hen’s eggs. Berl. Münch. Tierärzt. W. 115(5-6): 200-202.
Roberts, J.R. (2004) Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult Sci. 41(3): 161-177.
Roberts, J.R. (2008) Egg shell quality: any better, any worse? Proceedings of the World’s Poultry Congress, Brisbane 2008.
Roberts JR, Chousalkar K (2009) Egg quality testing and identification of quality risk factors. AECL report no UNE-86; Australian Egg Corporation Limited
Rodenburg, T.B., Tuyttens, F.A., Sonck, B., De Reu, K., Herman, L., Zoons, J. (2005) Welfare, health, and hygiene of laying hens housed in furnished cages and in alternative housing systems. J. Appl. Anim. Welf. Sci. 8: 211-226.
Ruhnke, I., Cowling, G., Sommerlad, M., Swick, R.A., Choct, M. (2015) Gut impaction in free range hens. In Proceedings of the 26th annual Australian Poultry Science Symposium. Poultry Research Foundation, University of Sydney: 26:242-244
Samiullah S., Roberts, J.R., Chousalkar, K.K. (2014) Effect of production system and flock age on egg quality and total bacterial load in commercial laying hens. J. Appl. Poult. Res. 23(1): 59-70.
Schwaiger, K., Schmied, E.M., Bauer, J. (2008) Comparative analysis of antibiotic resistance characteristics of Gram‐negative bacteria isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, Germany. Zoo. Publ. Hlth 55(7): 331-341.
Schoeters, G., Hoogenboom, R. (2006) Contamination of free‐range chicken eggs with dioxins and dioxin‐like polychlorinated biphenyls. Mol. Nutr. Food Res. 50(10): 908-914.
Sibanda TZ, Kolashyapati M, Boshoff J., Ruhnke I (2018) The effects of body weight on the diurnal range use and latency to use the range in free range laying hens. Proceedings of the Congress of the International Society for Applied Ethology, Canada.
Sibanda, T.Z., Walkden-Brown, S.W., Kolakshyapati, M., Dawson, B., Schneider, D., Welch, M., Iqbal, Z., Cohen-Barnhouse, A., Morgan, N.K., Boshoff, J., Ruhnke, I. (2019) Flock use of the range is associated with the use of different components of a multi-tier aviary system in commercial free-range laying hens. Br. Poult. Sci. 61(2): 97-106.
Sibanda, T.Z., Flavel, R., Kolakshyapati, M., Welch, M., Schneider, D., Ruhnke, I. (2020a) The association between range usage and tibial quality in commercial free-range laying hens. Br. Poult. Sci. https://doi.org/10.1080/00071668.2020.1759786
Sibanda, T.Z., Kolakshyapati, M., Welch, M., Schneider, D., Boshoff, J., Ruhnke, I. (2020b) Managing freerange laying hens – Part A: Frequent and non-frequent range users differ in laying performance but not egg quality. Animals 10:991 https://doi.org/10.3390/ani1006099.
Singh, M., Cowieson, A.J. (2013) Range use and pasture consumption in free-range poultry production. Anim. Prod. 53: 1202-1208.
Singh, R., Cheng, K. M., Silversides, F. G. (2009) Production performance and egg quality of four strains of laying hens kept in conventional cages and floor pens. Poultry Sci. 88(2), 256-264.
Siopes, T.D., Wilson, W.O. (1980) Participation of the eyes in the photo-stimulation of chickens. Poultry Sci. 59: 1122-1125.
Sirovnik, J., Würbel, H., Toscano, M.J. (2018) Feeder space affects access to the feeder, aggression, and feed conversion in laying hens in an aviary system. Appl. Anim. Behav. Sci. 198: 75-82.
Smith, S.F., Appleby, M.C., Hughes, B.O. (1990) Problem-solving by hens: opening doors to reach nest sites. Appl. Anim. Behav. Sci. 28: 287-292.
Snow, L.C., Davies, R.H., Christiansen, K.H., Carrique-Mas, J.J., Cook, A.J.C., Evans, S.J. (2010) Investigation of risk factors for Salmonella on commercial egg-laying farms in Great Britain, 2004–2005. Vet. Record. 166(19): 579-586.
Spliethoff, H.M., Mitchell, R.G., Ribaudo, L.N., Taylor, O., Shayler, H.A., Greene, V., Oglesby, D. (2014) Lead in New York City community garden chicken eggs: influential factors and health implications. Envir. Geo. Health, 36(4): 633-649.
Sulonen, J., Kärenlampi, R., Holma, U., Hänninen, M.L. (2007) Campylobacter in Finnish organic laying hens in autumn 2003 and spring 2004. Poultry Sci. 86(6): 1223-1228.
Tauson, R. (2002) Furnished cages and aviaries: production and health. Worlds Poultry Sci. J. 58(1): 49-63.
Tauson, R., Wahlström, A., Abrahamsson, P. (1999) Effect of two floor housing systems and cages on health, production, and fear response in layers. Appl. Anim. Behav. Sci. 8(2): 152-159.
Tserveni-Gousi, A.S. (2001) Sensory evaluation of eggs produced by laying hens fed diet containing flaxseed and thymus meal. Archiv für Geflügelk. 65(5): 214-218.
Tumova, E., Ebeid, T. (2003) Effect of housing system on performance and egg quality characteristics in laying hens. Sci. Agric. Bohem. 34: 73-80.
Van Den Brand, H., Parmentier, H.K., & Kemp, A.B. (2004) Effects of housing system (outdoor vs cages) and age of laying hens on egg characteristics. Br. Poult. Sci. 45(6): 745-752.
Van Horne, P.L.M. (1996) Production and economic results of commercial flocks with white layers in aviary systems and battery cages. Br. Poult. Sci. 37: 55-61.
Van Hoorebeke, S., Van Immerseel, F., Schulz, J., Hartung, J., Harisberger, M., Barco, L., Ricci, A., Theodoropoulos, G., Xylouri, E., De Vylder, J. and Ducatelle, R. (2010) Determination of the within and between flock prevalence and identification of risk factors for Salmonella infections in laying hen flocks housed in conventional and alternative systems. Prev. Vet. Med. 94(1-2): 94-100.
Van Overmeire, I., Pussemier, L., Hanot, V., De Temmerman, L., Hoenig, M., Goeyens, L. (2006) Chemical contamination of free-range eggs from Belgium. Food Add. Cont. 23(11): 1109-1122.
Valkonen, E., Venäläinen, E., Rossow, L., Valaja, J. (2010) Effects of calcium diet supplements on egg strength in conventional and furnished cages, and effects of 2 different nest floor materials. Poultry Sci. 89(11): 2307-2316.
Villanueva, S., Ali, A.B., Campbell, D.L., Siegford, J.M. (2017) Nest use and patterns of egg-laying and damage by 4 strains of laying hens in an aviary system. Poultry Sci. 96: 3011-3020. DOI: 10.3382/ps/pex104
Vincevica-Gaile, Z., Gaga, K., Klavins, M. (2013). Food and environment: trace element content of hen eggs from different housing types. APCBEE procedia, 5: 221-226.
Waegeneers, N., De Steur, H., De Temmerman, L., Van Steenwinkel, S., Gellynck, X., Viaene, J. (2009a). Transfer of soil contaminants to home-produced eggs and preventive measures to reduce contamination. Sci. Total Envir. 407(15): 4438-4446.
Waegeneers, N., Hoenig, M., Goeyens, L., De Temmerman, L. (2009b). Trace elements in home-produced eggs in Belgium: levels and spatiotemporal distribution. Sci. Total Envir. 407(15): 4397-4402.
Wales, A., Breslin, M., Carter, B., Sayers, R., Davies, R. (2007) A longitudinal study of environmental Salmonella contamination in caged and free-range layer flocks. Avian Pathol. 36(3): 187-197.
Wall, H. (2011). Production performance and proportion of nest eggs in layer hybrids housed in different designs of furnished cages. Poultry Sci. 90(10): 2153-2161.
Wall, H., Tauson, R. (2002). Egg quality in furnished cages for laying hens—Effects of crack reduction measures and hybrid. Poultry Sci. 81(3): 340-348.
Williams, A.G., Audsley, E., Sandars, D.L. (2006). Energy and environmental burdens of organic and non-organic agriculture and horticulture. Aspects of Applied Biology 79, What will organic farming deliver? COR 2006, 19-23.
Windhorst, H. W., Grabkowsky, B., Wilke, A. (2013) Atlas of the Global Egg Industry. International Egg Commission: London, UK.
Wolc, A., Arango, J., Settar, P., O’Sullivan, N.P., Dekkers, J.C. (2011) Evaluation of egg production in layers using random regression models. Poultry Sci. 90: 30-4.
Zhao, Y., Shepherd, T.A., Li, H., Xin, H. (2015) Environmental assessment of three egg production systems–Part I: Monitoring system and indoor air quality. Poultry Sci, 94(3): 518-533.
اشترك في نشرتنا الإخبارية
وتعرّف على آخر أخبار الصناعة.