Summary
The growing World population and limited natural resources require a more efficient utilization and
conversion of resources in available phytogenic biomass. In the future there will be a very strong
competition for arable land or phytogenic biomass resp. between food/feed, fuel, fibre and further
industrial materials as well as areas for settlements and natural conservation. Therefore plant breeding
should focus on high yielding plants with low external inputs (Low Input Varieties). Apart from traditional
plant breeding, plant biotechnology may contribute to this objective.
Presently, we are in an initial phase of this breeding technology. The cultivation of genetically modified
plants (GMP) increased from 1.7 (1996) to about 148 million ha (2010), i.e. about 10% of total arable
land. Most modified cultures are soybean, maize, cotton and rapeseed, mainly with increased tolerance
against herbicides and insecticides or higher resistance against insects.
Safety and nutritional assessment of food/feed from GMP is urgently necessary. Strict regulations for
these assessments exist in many countries. The results of the nutritional studies are summarized in
this review. Up to now more than 1 billion ha of GMP have been cultivated all over the world. Nutritional
assessment starts with compositional analysis followed by digestion and feeding studies, fates of
transgenic DNA and newly expressed proteins. Up to now most studies were done with GM-crops of
the 1st generation (plants with input traits; without substantial changes in composition). No unintended
effects in composition or contamination (except lower concentration of mycotoxins) and nutritional
assessment of feeds from GM-crops of the 1st generation were registered in about 150 scientific
studies with food producing animals. Most of the studies were done with broilers. Transgenic DNA
and newly expressed proteins did not show other properties as plant DNA or native plant proteins
during feed treatments or in the animals.
Other experimental designs for nutritional and safety assessment are recommended for GM-plants with
output traits or with substantial changes in composition (plants of the 2nd generation).

Introduction
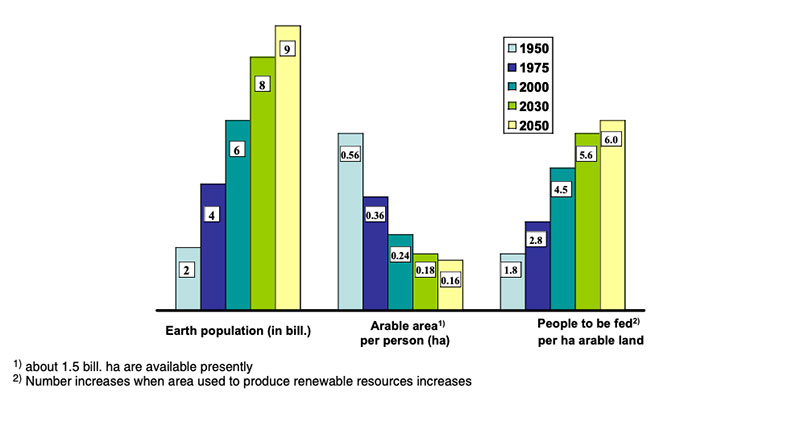
The production of high amounts of phytogenic biomass with high quality or high bioavailability of valuable nutrients is one of the most important challenges to meet future demand (SCAR 2008; Flachowsky 2008; The Royal Society 2009). The world population is predicted to grow from presently 7 billion to about 9 billion in 2050, and the demand for food of animal origin may double (Steinfeld et al. 2006; Godfray et al. 2010), driven by increasing income from productive employment (Keyzer et al. 2005) and preference for “Western style of life” in many developing countries. Food of animal origin like poultry meat and eggs contributes to meet the human requirements in amino acids and many trace nutrients. The production of food of animal origin requires vast resources (e.g. Flachowsky 2002, 2011) especially in terms of arable land for feed production. Figure 1 shows the effects of population growth on the availability of arable land per person and the number of people to be fed per ha arable land during the time from 1950 to 2050. Furthermore, feed/food production causes emissions with greenhouse gas potential such as carbon dioxide (CO2) from fossil fuel, methane (CH4) from the enteric fermentation (esp. ruminants) and from the excrement management as well as nitrogen-compounds (NH3, N2O) from the protein metabolism in the animals (see DEFRA 2006; Flachowsky and Hachenberg 2009, FAO 2010; Grünberg et al. 2010; Leip et al. 2010). Additional arable land will be needed to produce biofuel and material for the industry, competing with land use for feed production. Therefore plant breeding and cultivation are the focal points for globalFigure 1: Population growth, arable land area available per person and number of people to
be fed per ha(according to FAO yearbooks)
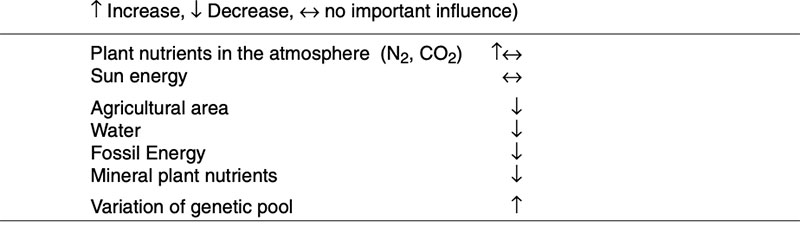
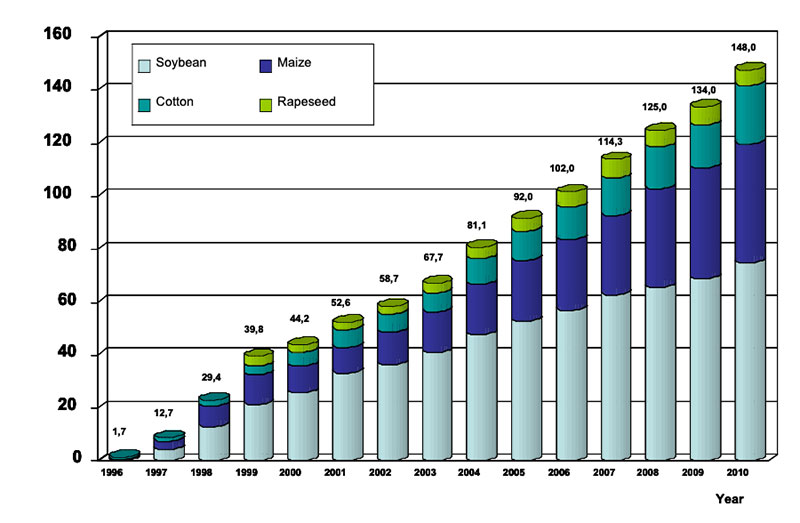
feed and food security in the years ahead. High yielding plants with low external inputs of limited natural resources should be the main goals of plant breeding in the future. So-called “Low Input Varieties” should use unlimited resources such as sunlight or sun energy, nitrogen (N2) and CO2 as plant nutrients from the atmosphere to the highest possible level and should use limited resources such as agricultural area, water, fossil energy, phosphorus etc. as effectively as possible (see Table 1). The biodiversity of microorganisms, plants and animals offers an extremely large gene pool which has been already used by traditional plant breeding and which could be used more intensively in the future. Apart from traditional breeding, plant biotechnology apparently has a potential to contribute to the objective of “Low Input Varieties”. The cultivation of GMP increased worldwide from about 1.7 (in 1996) to nearly 148 million ha (in 2010), representing about 10% of arable land (James 2011). In % of the global GM area, the most important GM-crops are currently soybeans (60), corn (24), cotton (11) and canola (5) (Figure 2).
Table 1: Potentials to produce phytogenic biomass and their availability per inhabitant with
increasing of population (Flachowsky 2010)
Definitions
-
The most important objectives for plant breeders are:
- High yields with low external inputs (Low Input Varieties) of limited resources (see Table 1)
- Lower concentrations of anti-nutritive (toxic) substances such as secondary plant products, mycotoxins, toxins from anthropogenic activities or inhibitors (e.g. phytate, lignin)
- Higher concentration of the components determining nutritive value such as nutrient precursors, nutrients, enzymes, prebiotics, essential oils etc.
Presently, most of the Genetically Modified Plants (GMP) are modified for agronomic traits (see Figure 2) such as increased tolerance against insects or higher resistance against insecticides or pesticides. Such plants are characterized by so-called input traits (GMP of the first generation) without substantial changes in composition and/or nutritive value. Such plants can be considered as substantially equivalent to their isogenic counterpart (OECD 1993).
Figure 2: Global area of transgenic crops (James 2011)
GMP of the second generation (with output traits) should contain more nutrients or less anti-nutritive substances. Such plants (feeds) are not substantially different in composition from their counterpart. GMP offer a wide range of application in animal nutrition. Seeds and by-products from food and biofuel industry are the most important feedstuffs for poultry.
Based on the present (public) situation animal nutritionists are to address the following aspects:
- Nutritional and safety assessment of feed from the 1st generation of GMP
- Nutritional and safety assessment of feed from the 2nd generation of GMP
- Influence of GM-feed on animal health and quality of food of animal origin
- Studies on the behaviour/degradation of newly expressed (novel) proteins, foreign DNA, side effects etc.
In Europe the safety of GMP for humans, animals and the environment is assessed by the Panel for Genetically Modified Organisms (GMO-Panel) of the European Food Safety Authority (EFSA, located in Parma; Italy), based on various Guidance documents (e.g. EFSA 2006, 2008). The EU-Commission is responsible for the risk management.
Compositional analysis
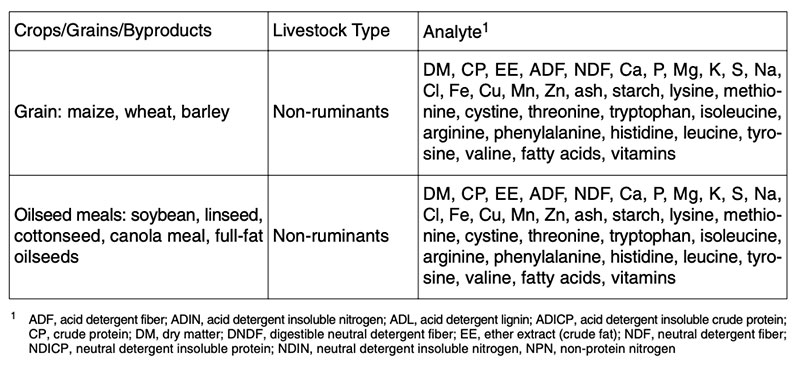
Composition analysis of feeds from GMP is the starting point for nutritional assessment. There are different recommendations for compositional analysis of GMP for feed groups (e.g. concentrates, forages etc.) and for animal groups (e.g. ruminants and non-ruminants), as shown for non-ruminants in Table 2. Between 60-100 ingredients of transgenic, isogenic and commercial varieties will be determined to compare the composition of plants and feeds from plants. In addition the newly expressed protein(s) and their degradability (mostly in vitro) will be determined.No additional animal studies are recommended (EFSA 2006, 2008) if the GMP are substantially equivalent to their isogenic counterpart in the case of GMP of the 1st generation. Nevertheless many feeding studies with feeds from GMP of the 1st generation have been carried out during the last few years. Incidentally, all these studies can contribute substantial information to feed science, which has been dramatically neglected during the last 30 years.
Table 2: Examples for recommendations of compositional analysis of feeds from GMP,
isogenic counterparts and commercial varieties for non-ruminants (see ILSI 2007 and
OECD 2001-2005)
Feeding studies
Types of studiesDetails of sampling for animal feeding studies, handling of samples and preparation of samples for animal feeding studies are described by ILSI (2007) and are shown in Figure 3. Feeding studies with laboratory animals and with food producing (target) animals can be done with various objectives to answer different questions (Table 3; see also Flachowsky and Wenk 2010). Many studies were done with laboratory animal models for toxicity testing of single substances (single dose toxicity testing, repeated dose toxicity testing, reproductive and development toxicity testing, immunotoxicity testing etc.; EFSA 2008). Laboratory animals were also used for the safety (and nutritional) assessment of the whole GM-food and feed (in general 90-day feeding studies to detect unintended effects, sub-chronic animal tests, allergenicity tests; for margins of safety etc.; EFSA 2008; 2011; OECD 1995).
Studies with target animals are more of nutritional concern. The type of study depends on the type of genetic modification in plants, the availability of GM-feed and further factors (see Tables 3 and 4).
Figure 3: Flow diagram for animal feeding studies (by ILSI 2007)
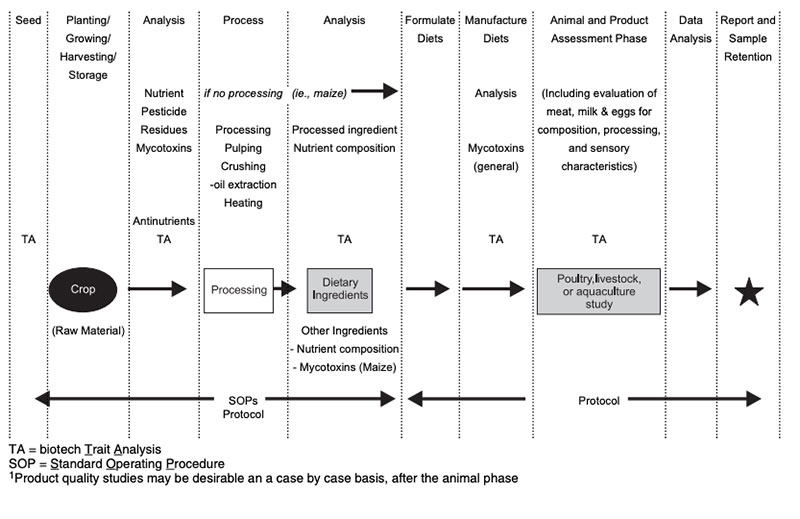
Table 3: Important types of feeding studies with animals for safety and nutritional assessment
of feed from GMP
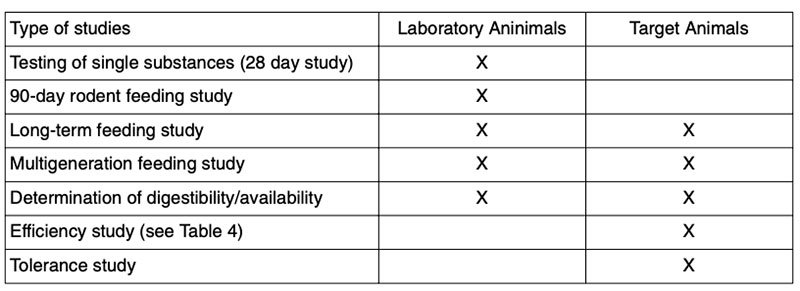
Table 4: Examples of life spans for poultry in efficacy studies
(in days; adapted from ILSI 2003, 2007)
Results of feeding studies with GMP of the 1st generation
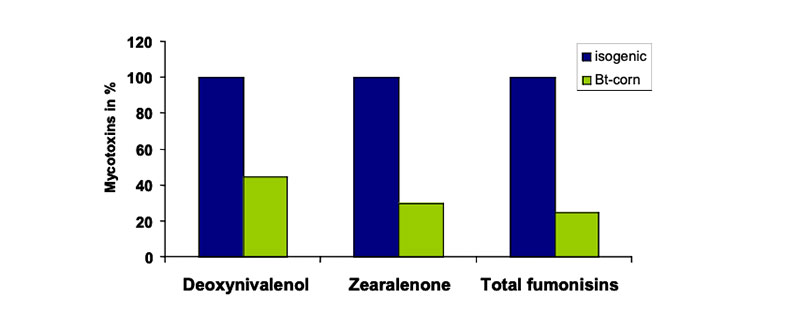
In previous studies the authors compared only the composition and the nutritive value of one feed (e.g. transgenic origin) with another one (e.g. isogenic counterpart) and neglected the considerable biological range described e.g. in the OECD-consensus documents (OECD 2001a, 2001b, 2002a, 2002b, 2003, 2004a, 2004b, 2004c, 2005) or other feed value tables. In general GMP´s of the first generation were essentially equivalent to their isogenic counterparts. Under some cultivation conditions the mycotoxin contamination of GMP feed was lower than in feed from non-GM plants. For example, Bt maize is less severely attacked and weakened by the European corn borer and might have a greater resistance to field infections, particularly to Fusarium fungi, which produce mycotoxins. Evidence of reduced mycotoxin contamination in GMP has been demonstrated in some, but not all studies, as summarized by Flachowsky et al. (2005a). In long-term studies, numerous researchers investigated the influence of levels of corn borer infestation of isogenic and Bt hybrids on mycotoxin contamination. Most researchers reported a lower level of mycotoxin contamination in the transgenic hybrids, over a considerable geographical and time range of observations (Figure 4).Figure 4: Mycotoxins in isogenic (100 %) and Bt-corn (% of isogenic corn; Sources: Bakan et
al. 2002, Cahagnier and Melcion 2000, Munkvold et al. 1999, Pietri and Piva 2000,
Reuter et al. 2002, Valenta et al. 2001)
In early feeding studies with food producing animals, feeds from GMP of the first generation were only compared with their isogenic counterparts to demonstrate equivalence (OECD 1993). Later studies included three or more commercial varieties to measure also the biological range of various measurements. In recent years about 150 feeding trials with food producing animals were reported in peer reviewed papers and summarized in several reviews (see above).
The inclusion of commercial varieties in such studies as recommended by ILSI (2007) and EFSA (2008) may contribute to a more biologically relevant assessment of the results of animal feeding studies (e.g. Lucas et al. 2007; McNaughton et al. 2007; see Table 5).
Table 5: Effect of GM maize DAS-59122-7 (53 to 70% maize in the diet) on broiler performance
compared to the near isogenic control and three non-GM hybrids
(McNaughton et al., 2007; 120 broilers per treatment)
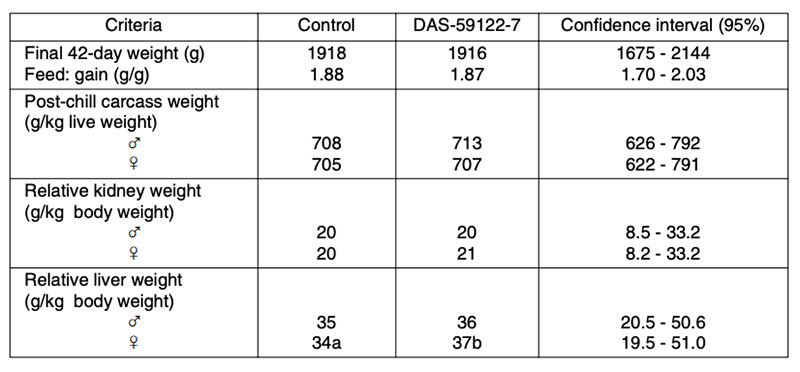
Apart from a statistically significant small increase in relative liver weight (p<0.05) of female broilers, no relevant differences between transgenic maize (DAS-59122-7) and its isogenic counterpart were found in this feeding trial. The inclusion of several commercial non-GM-varieties in the field and in animal feeding studies should help to avoid wrong conclusions from experimental data. Long term feeding studies cover a very long period of the life or the whole lifespan of the animals. Results from such studies and multi-generation studies may include not only the animals´ growth performance, but also their health and reproductive performance (BEETLE 2009) in response to being fed high amounts of GM-feed. In laboratory studies, no negative effects on reproductive traits were found in rodents fed with Bt-corn, glyphosate tolerant soybeans or GM-potatoes compared with their conventional counterparts (Brake and Everson 2004; Kilic and Akay 2008; Rhee et al. 2005). The results of two multi-generation studies at our Institute with laying hens (Halle et al. 2006) and quails (Figure 5) showed no differences in production and reproduction performance between laying quails fed diets containing 50% Bt maize vs. diets containing 50% isogenic maize Table 6 summarizes results from feeding trials with different poultry species and categories, comparing feeds of GMP of the first generation (plants with input traits) with their isogenic counterparts. The absence of biologically relevant adverse effects in poultry studies is not surprising in view of the compositional equivalence between feeds from isogenic and transgenic plants and the general observation that GMP of the 1st generation are comparable with plants from traditional breeding.
Figure 5: (A) Body weight of female quails (age: 6 weeks), (B) laying intensity and (C)
hatchability of quails fed with isogenic (black columns) and transgenic (Bt, white
columns) maize in a 10 generation experiment (Flachowsky et al. 2005b)
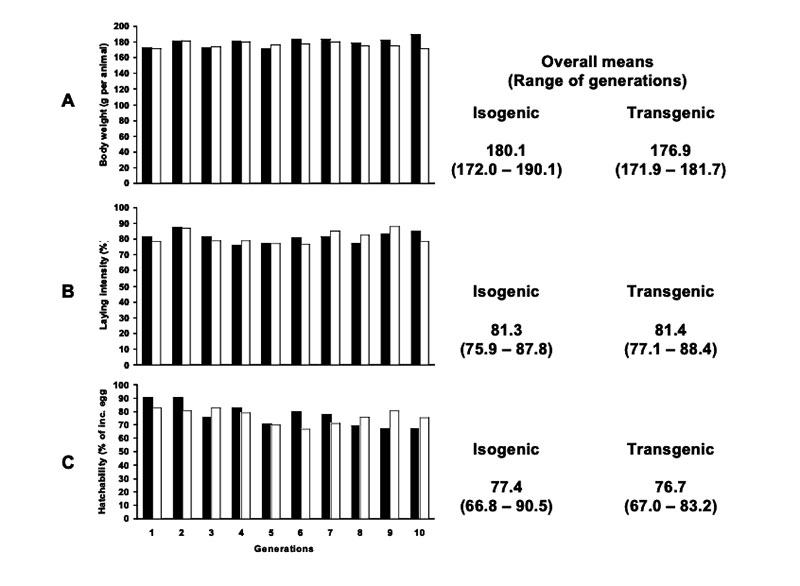
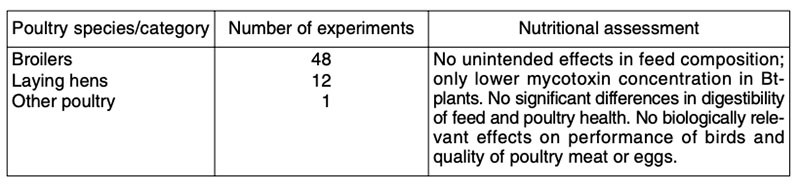
Table 6: Published comparisons of feeds from first generation GMP (mainly maize, soybeans,
cotton, canola) of various constructs with their isogenic counterparts
Results of feeding studies with GMP of the 2nd generation
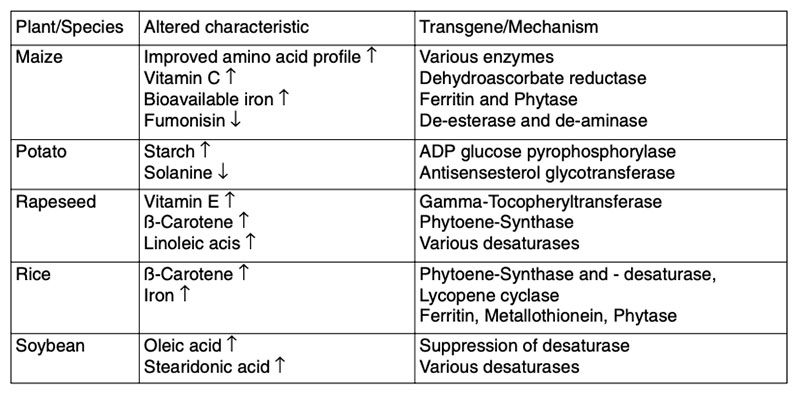
During the last few years much attention has been spent to develop GMP, in which significant intended alterations in composition have been achieved in order to enhance the nutritional properties or health benefits. Examples of nutritionally improved GMP are given in Table 7.Table 7: Examples of GMP with improved characteristics intended to provide nutritional
benefits (EFSA 2008)
New experimental designs are necessary for nutritional assessment of GMP of the 2nd generation (Flachowsky and Böhme 2005; ILSI 2007; EFSA 2008, 2011; Flachowsky and Wenk 2010) to test the significance of higher concentrations of valuable substances such as nutrients or nutrient precursors or lower concentrations of undesirable ingredients. An experimental design to demonstrate the bioavailability of a nutrient precursor is shown in Table 8.
Table 8: Examples for nutritional assessment of 2nd generation GMP (GM-plants with output
traits, e.g. higher concentration of the vitamin A precursor ß-carotene
(EFSA 2008)
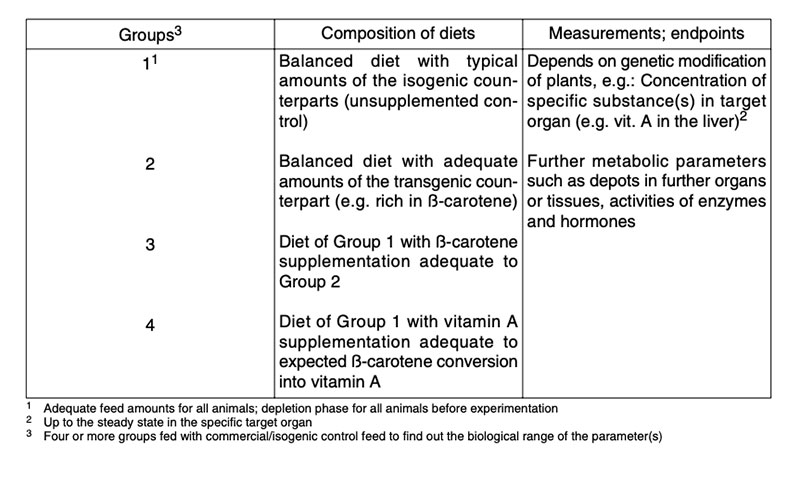
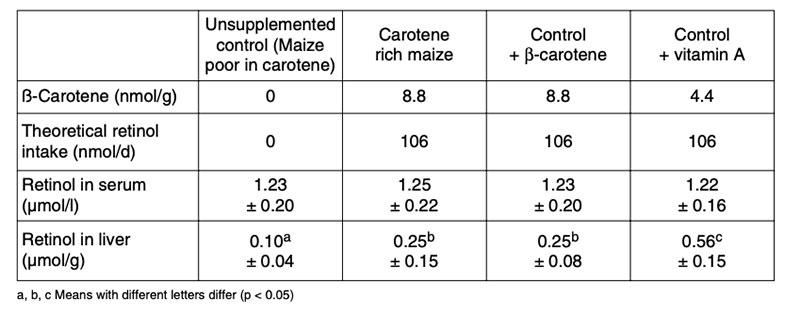
Table 9 shows an example to determine the ß-carotene conversion from maize into vitamin A in Mongolian gerbils.
Table 9: Experimental design to assess the conversion of β-carotene into vitamin A in
Mongolian gerbils (60% maize in diets; n = 10, depletion phase: 4 weeks, feeding:
8 weeks; Howe and Tanumihardjo 2006)
Adequate studies are necessary to demonstrate the effects of other newly expressed nutrients or higher levels of nutrients such as amino acids (Lucas et al. 2007), fatty acids (Meja et al. 2010), nonessential substances like enzymes or essential oils (Zhang et al. 2000).
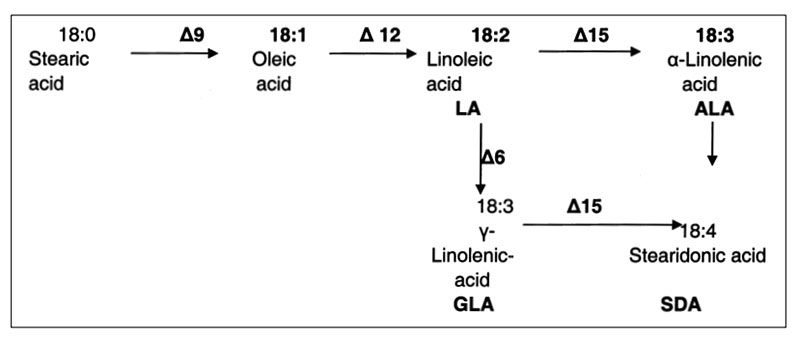
The introduction of new gene fragments may trigger the expression of new substances, which were never before in such plants. A recent example is the introduction of genes which express two desaturases in soybeans with the consequence to synthesize C18:4 n-3 octadecatetraenoic acid, also known as stearidonic acid (SDA; see Figure 6). This long-chain omega-3 fatty acid is one of the precursors for the formation of the long chain omega-3 polyunsaturated fatty acids 20:5 n-3 eicosapentaenoic acid (EPA) and 22:6 n-3 docosahexaenoic acid (DHA) which are essential for human and animal nutrition and have potential health benefits (Gebauer et al. 2006, Ursin 2003; Harris et al. 2008, Whelan et al. 2009).
The SDA-content of such soybean oil may vary between 20 and 30%. Rymer et al. (2011) added 45 (grower) and 50g (finisher) soybean oil containing 24% SDA to broiler feed and confirmed results from lactating cows (Bernal-Santos et al. 2010): increased concentration of SDA, EPA and DHA in various meat samples, compared to conventional soybean oil. Even higher EPA and DHA concentrations were achieved with fish oil supplementation, but the fishy taste was not acceptable. Gibbs et al. (2010) suggested the int roduction of SDA in broiler feed as a possibility to increase the longchain n-3 PUFA intake of humans.
Fate of transgenic DNA and newly expressed proteins
The intake of feeds from GMP results in the ingestion of transgenic DNA and newly expressed protein(s). Several studies were conducted to trace their fate during food/feed processing and when passing through the gastrointestinal tract of animals, and the extent to which transgenes or their products may be incorporated into animal tissues. Table 10 shows the influence of various processing conditions on some DNA fragments of rapeseed. Higher temperatures and extraction contributed to the degradation of DNA fragments. There is agreement among authors (Mazza et al. 2005, Sharma et al. 2006, Alexander et al. 2007) that recombinant DNA would be processed during feed treatment (ensiling, extraction etc.; see Table 10) and in the gut in the same manner as genetic material from endogenous feed, as shown in feeding studies with non-ruminants at our Institute (see Table 11) andFigure 6: Synthesis of Stearidonic acid (C18:4n) in genetically modified soybeans and the
effects of various desaturases (from Ursin 2003 und Whelan 2009)
several other institutions. Small DNA fragments from isogenic and transgenic plants could be detected in blood, spleen, liver and kidney (Mazza et al. 2005).
Table 10: Processing of rapeseed for oil production and DNA fragments determined in final
products of isogenic (i) and transgenic (t) rapeseed (Berger et al. 2003)
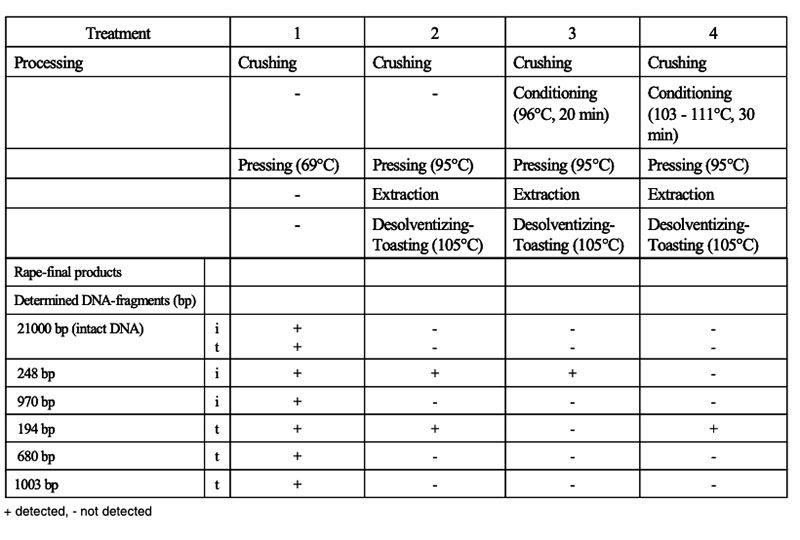
Table 11: Studies of the Institute of Animal Nutrition, FLI, on transfer of DNA fragments in
food producing animals
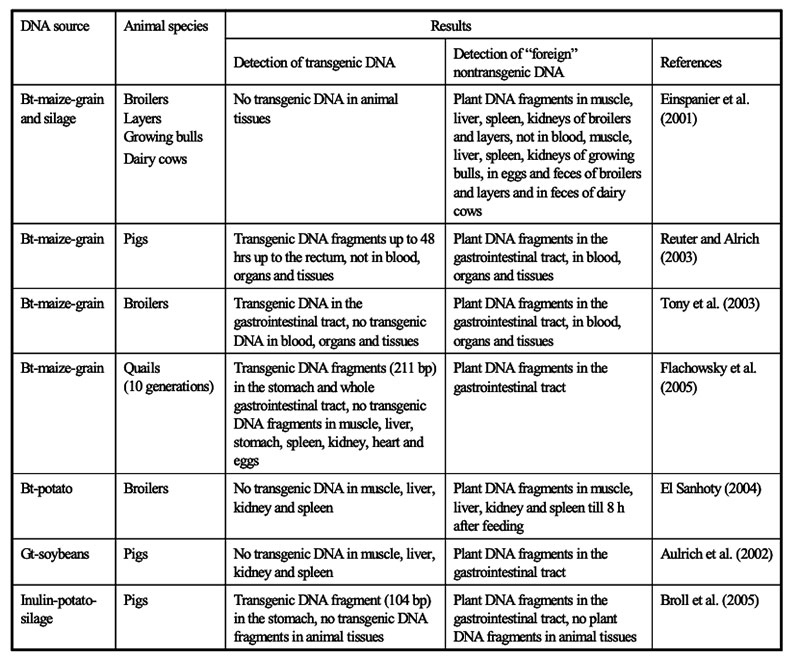
Newly expressed proteins show similar chemical and physiological properties, including microbial and enzymatic degradation (Hammond 2008), as native plant proteins (Alexander et al. 2007).
Future tendencies
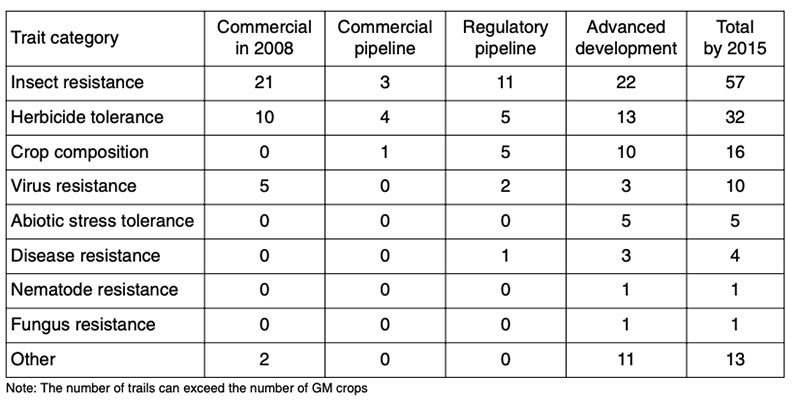
Presently many GMP containing stack events are being developed and already in cultivation (Figure 7). That means for example, the plants are resistant against insects and tolerant against insecticides. There are already plants in the pipeline containing up to eight stacks. In the future we may expect GM-plants with changed composition (2nd generation of GMP), more resistant against biotic and abiotic stressors such as drought and saline soils and more efficient in using limited natural resources (Low Input Varieties; see Table 12).
Figure 7: Global area cultivated with the main GM traits
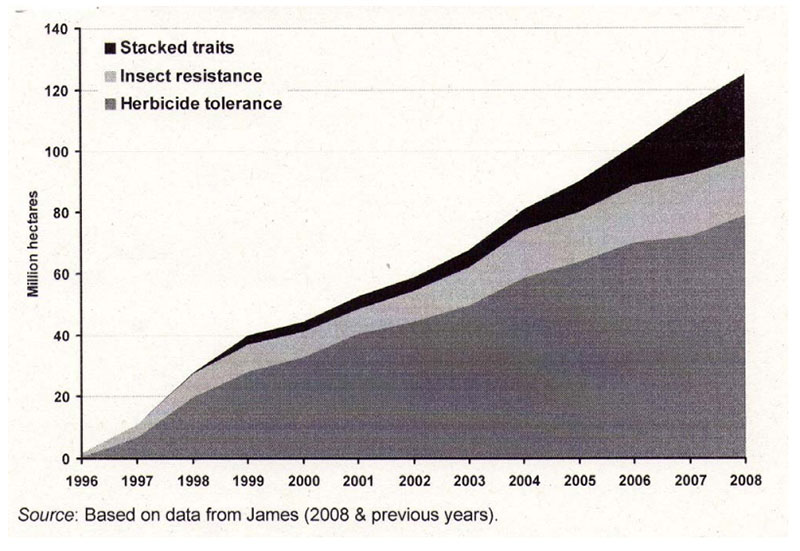
Table 12: Present situation and future tendencies in global cultivation of GMP (Stein and
Rodriguez-Cerezo, 2009)
Conclusions
“Green” biotechnology should be considered as a method of plant breeding. Presently, the breeders improve resistance and tolerance of plants against insects, herbicides and/or insecticides (plants of the 1st generation) or influence the composition of GMP by increasing valuable nutrients and/or decreasing anti-nutritive substances (plants of the 2nd generation). Many new developments, including changes in composition, are in the pipeline by different companies. Furthermore, GMP´s are being developed to improve their agronomic properties such as drought resistance and salt tolerance (abiotic stressors; see Table 12).Assessing the nutritive value and the safety of feeds from plant breeding and dealing with GM-animals are real challenges for animal nutritionists in the future (Figure 8). Various types of studies are necessary to answer all the questions and to contribute to a better public acceptance of such plants and animals (see Tables 3, 4, 8 and 9).
Figure 8: Animal nutrition (nutritional assessment of feeds) between plant and animal breeding
Presently, 10% of the global arable land is cultivated with GM-plants of the first generation, which have been tested in about 150 feeding studies with food producing animals. No biologically relevant effects have been described in peer reviewed papers where the authors compared feed from GMP with their isogenic counterpart and commercial varieties if fed to broilers or other food producing animals.
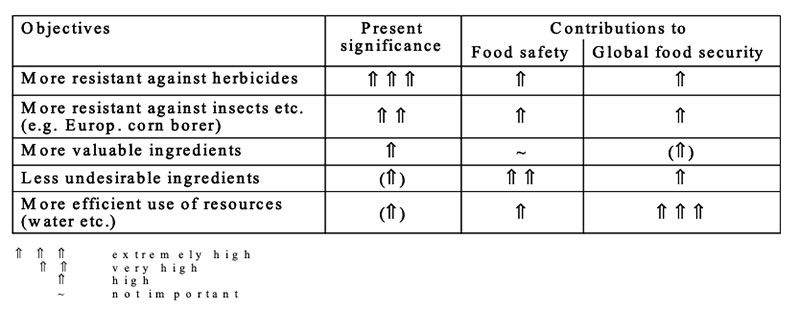
GMP for more efficient use of limited resources such as water, arable land, fertilizers etc. are under development (see Table 12), but not yet in cultivation. Development of such plants is a real challenge for plant breeders all over the world for substantial contributions to global food security (Table 13). Safety and nutritional assessment of GMP and feeds from GMP are a substantial prerequisite for feeding such products to food producing animals and for a better acceptance in the society
Table 13: Assessment of present modifications of plants from the view of food safety and
food security
References
Alexander T.W., Reuter T., Aulrich K., Sharma R., Okine E. K., Dixon W.T., McAllister T.A. (2007): A review of the detection and fate of novel plant molecules derived from biotechnology in livestock production. Anim. Feed Sci. Technol. 133: 31-62.Aulrich, K., Reuter, T., Flachowsky, G. (2002): The fate of foreign DNA in farm animals fed with genetically modified plants. Proc. Soc. Nutr. Physiol. 11: 187-188.
Aumaitre A., Aulrich K., Chesson, A., Flachowsky, G., Piva, G. (2002): New feeds from genetically modified plants: Substantial equivalence, nutritional equivalence, digestibility, and safety for animals and the food chain. Livest. Prod. Sci., 74, 223- 238.
Bakan, B., Melcion, D., Richard-Molrad, D., Cahagnier, B. (2002): Fungal growth and fusarium mycotoxin content in isogenic traditional maize and genetically modified maize grown in France and Spain. J. Agric. Food Chem. 50: 728-731.
BEETLE (2009): Long-term effects of genetically modified (GM) crops on health and the environment (including biodiversity). Executive Summary and Main Report. Federal Office of Consumer Protection and Food Safety (BVL), Berlin, 132 p., + 6 Annexes
Berger, B., Aulrich, K., Fleck, G., Flachowsky, G. (2003): Influence of processing of isogenic and transgenic rapeseed on DNAdegradation. Proc.Soc.Nutr.Physiol. 12: 108 (Abstract)
Bernal-Santos B., O´Donell S.M., Vicini J.L., Hartnell G.F., Bauman D.E. (2010): Hot topic: Enhancing omega-3 fatty acids in milk fat of dairy cows by using stearidonic acid-enriched soybean oil from genetically modified soybeans. J.Dairy Sci. 93: 32-37.
Brake, D.E., Everson, D.P. (2004): A generational study of glyphosate-tolerant soybeans on mouse fetal, postnatal and adult testicular development. Food Chem. Toxicol. 42: 29-36.
Broll, H., Zagon, J., Butschke, A., Leffke, A., Spiegelberg, A., Böhme, H., Flachowsky, G. (2005): The fate of DNA of transgenic inulin synthesizing potatoes in pigs. J. Anim. Feed Sci. 14, Suppl. 1: 337-340.
Cahagnier, B., Melcion, D. (2000): Mycotoxins de Fusarium dans les mais – grains a la recolte: relation entre la presence d’insects (pyrale, sesamie) et la teneur en mycotoxins. Proc. 6th Int.. Feed Proc. Conf., 27-28 November, Piacenca: 237- 249.
CAST (2006): Safety of meat, milk, and eggs from animals fed crops derived from modern biotechnology. No 34, 8 p.
Chesson, A., Flachowsky, G. (2003): Transgenic plants in poultry nutrition. World´s Poultry Sci. J. 59: 201-208.
Clark, J.H., Ipharraguerre, I.R. ( 2001) Livestock performance: Feeding biotech crops. J. Dairy Sci. 84: Suppl. E9-E18, 237- 249.
Clark, J.,H., Ipharraguerre I.,R. (2004): Biotechnology crops as feeds for livestock. In Agricultural Biotechnology challenges and Prospects by M.K. Balgat et al. (eds.), American Chem. Soc., Washington D.C., 177-198.
DEFRA (2006): Dtermination the environmental burdens and resource use in the production of agriculture and horticulture commodities. Defra project report 205, Cranfield, Univ. Silsoe Inst., http://www.cranfield.ac.uk.
EFSA (2006): Guidance document of the Scientific Panel on Genetically Modified Organisms for the risk assessment of genetically modified plants and derived food and feed. EFSA Journal 99, 1-100.
EFSA (2008): Safety and nutritional assessment of GM plant derived food and feed. The role of animal feeding trials. Food and Chemical Toxicology 46, 2-70.
EFSA (2011): Guidance on the safety assessment of genetically modified animal-derived food and feed (in preparation) Einspanier, R., Klotz, A., Kraft, J., Aulrich, K., Poser, R., Schwägele, F., Jahreis, G., Flachowsky, G. (2001): The fate of forage plant DNA in farm animals: A collaborative case-study investigating cattle and chicken fed recombinant plant material. Eur. Food Res. Technol., 212: 129-134.
El Sanhoty, R.M.E.S. (2004): Quality control for food produced by genetic engineering. Diss., TU Berlin, 155 pp.
EU (2000): Weißbuch der Lebensmittelsicherheit. Brüssel, 12.01.2000, ISSN 0254-1467, 66 S.
FAO (2010): Greenhouse gas emissions from the daity sector. A life cycle Assessment, Food and Agriculture Organisation of the United Nations. Rome, 90 p.
Flachowsky G. (2008): What do animal nutritionists expect from plant breeding? Outlook on Agriculture. 37: 95-103.
Flachowsky, G. (2010): Global food security: Is there any solution? (in German), NovoArgumente 105: 64-68.
Flachowsky, G. (2011): Feed from genetically modified plants in poultry nutrition. Proc. 18th European Symp. on Poultry, 31.10.-04.11.2011; Cesme/Izmir; Turkey, (in press).
Flachowsky G., Aulrich K., Böhme H., Halle I. (2007): Studies on feeds from genetically modified plants (GMP) Contributions to nutritional and safety assessment. Animal Feed Science Technology 133: 2-30.
Flachowsky G., Böhme H. (2005): Proposals for nutritional assessments of feed from genetically modified plants. J. Anim. Feed Sci. 14: 49-70.
Flachowsky G., Chesson A., Aulrich K. (2005): Animal nutrition with feeds from genetically modified plants. Archiv Anim. Nutr. 59: 1-40.
Flachowsky, G., Hachenberg, S. (2009): CO2-footprints for food of animal origin – Present stage and open questions. J. Consumer Prot. and Food Safety 4, 190-198.
Flachowsky, G., Halle, I., Aulrich, K. (2005b): Long term feeding of Bt-corn – a ten generation study with quails. Arch. Anim. Nutr. 59: 449-451.
Flachowsky G., Wenk C. (2010) The role of animal feeding trials for nutritional and safety assessment of feeds from genetically modified plants – Present stage and future challenges Journal Animal Feed Sciences 19: 149-170.
FSANS ( 2007) The role of animal feeding studies in the safety assessment of genetically modified foods. Rep. Workshop of Food Standards Australia New Zealand, 15th June 2007, Canberra, 19p.
Gebauer S.K., Psota T.L., Harris W.S., Kris-Etherton P.M. (2006) n-3 Fatty acid recommendations and food sources to achieve essentiality and cardiovascular benefits. American Journal Clinical Nutrition 83(Suppl.) 1526S-1535S.
Gibbs, R.A., Rymer, C., Givens, D.I. (2010): Long-chain n-3 PUFA intakes in the UK and the potential of a chicken meat prototype to increase them. Proc. Nutr. Soc. 69, 144-155.
Godfray, H.C., Beddington, J.R., Crute, I.R.,, Haddad, L., Wawrence, D., Muir, J., Pretty, J., Robinson, S., Thomas, S.M.,
Toulmin, C. (2010): Food security: The challenge of feeding 9 billion people. Sci. 327: 812-818.
Grünberg, J., Nieberg, H., Schmidt, T. (2010): Treibhausgasbilanzierung von Lebensmitteln (Carbon Footprints): Überblick und kritische Reflektion. Landbauforschung – vTI Agriculture and Forestry Research 60, 63-70.
Halle, I., Aulrich, K., Flachowsky, G. (2006): Four generatios feeding of GMO-corn to laying hens. Proc. Soc. Nutr. Physiol. 15: 114 (Abstr.).
Harris W.S., Lemke S.L., Hansen S.N., Goldstein D.A., DiRienzo M.A.,Su H., Nemet M.A., Taylor M.L., Ahmed G., George C. (2008): Stearidonic acid-enhanced soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipds 43, 805-811.
Howe J.A., Tanumihardjo S.A., (2006): Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian Gerbils. J. Nutr. 136, 2562-2567.
ILSI (2003): Best practices for the conduct of animal studies to evaluate crops genetically modified for input traits. International Life Sciences Institute, Washington, D.C. 62 p. http//www.ilsi.org/file/bestpracticescas.