Introduction
Bacterial infections are of great economic importance in the poultry industry worldwide. Particularly in the chicken layer industry recent reports of colibacillosis, erysipelas and fowl cholera in Western Europe implicate a resurgence of these diseases (ZANELLA et al. 2000, VANDEKERCHOVE et al.2004, MAZAHERI et al. 2006). This might be attributed to a change from cage housing systems to alternative housing systems like aviaries and free-range systems and an accompanied increase of infectious pressure (MAZAHERI et al. 2006).
To prevent bacterial diseases in the egg production industry, vaccination is of increasing relevance as it encounters problems due to growing antibiotic resistance and an increased consumer demand concerning food safety (VAN OIRSCHOT 1994, WHITE et al. 2002). Furthermore, vaccination can avoid withdrawal periods in marketing eggs following antibiotic treatment. As commercial vaccines are frequently not efficient due to the high antigenic variability of the disease causing organism (BRAGG, R.R. 2002, SMART et al. 1993) or as commercial vaccines are not available for certain diseases like erysipelas in chickens, autogenous vaccines can offer sophisticated alternatives.
Adjuvants used in vaccine preparations for layers are in general either aluminium hydroxide or different mineral oils in different phase combinations. Each adjuvant has advantages and disadvantages. An aluminium hydroxide gel adjuvant vaccine usually causes little local reaction and the antibody titre increases quite rapidly after injection (MOREIN et al. 1996). Unfortunately, this titre also decreases rapidly in almost the same manner, thus requiring a second injection. A mineral oil adjuvant will release the antigen slowly and respond directly to antigen-presenting macrophages, resulting in antibodies being able to have a longer persistence than aluminium hydroxide gel adjuvant vaccine. Thus, often only a single application is required. On the other hand, the oil responsible for the slow release might cause local reactions (DEGUCHI et al., 1998, FUKANOK et al., 2000, MOREIN et al., 1996, REID et al. 1987).
Field records from layers have been used to estimate the effect of different adjuvants on the protection level after vaccinating against E.coli during rearing.
We conducted a retrospective analysis of field records to elucidate the influence of the adjuvant in the autogenous vaccine on hen-housed production and mortality, based on weekly records from five farms with up to 9 years of data per farm. Differences due to the autogenous vaccine were estimated from a total of 81 flocks, using variance analysis with the program package SAS (SAS, 2004).
Material and Methods
Farms, housing systems and genotypes The data included in the analysis were recorded on five farms of different egg producers. Each farm had two separate houses with up to nine flocks with complete weekly records for this study. The number of hens per flock varied between about 8,000 and 43,000 hens with an average of 20,000 hens per flock. Two farms were equipped with aviaries, three used free-range systems. Three different genotypes, two brown and one white-egg layer strain were included in the study. Since the type of layer was highly confounded with farm and housing system, the genotype was not considered in further analysis. Each house was treated as separate experimental unit. The general time trend was measured as linear regression.Autogenous Vaccines
Each flock was vaccinated during rearing with a trivalent autogenous vaccine containing relevant strains of the bacterial pathogens Escherichia coli, Pasteurella multocida and Eryselpelothrix rhusiopathiae.The last two flocks per farm were vaccinated intramuscularly once with an autogenous vaccine produced by Lohmann Tierzucht (LTZ) at an age of 14 weeks, following several flocks on the same farms vaccinated twice with an autogenous vaccine produced by another company at 14 and 16 weeks of age. The LTZ vaccine was blended with mineral oil as adjuvant and the other vaccine used aluminium hydroxide as carrier substance to enhance the immune response.
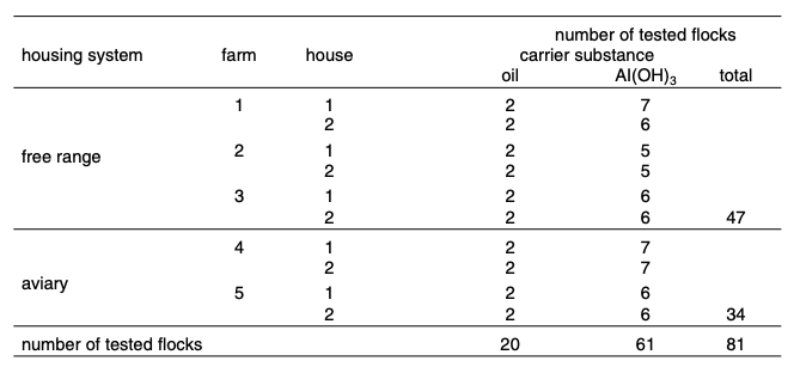
The distribution of flocks over system, farm, house and vaccination programs in rearing is shown in table 1.
Table 1: Number of tested flocks per environment, farm, house and type of vaccine
Statistical Analysis
The weekly records for mortality and rate of lay from 22 to 69 weeks of age were combined into twelve 4-week periods for analysis purposes. To estimate the time trend, consecutive flocks in each house were assigned a serial number, which was included in the model. In total, up to 12 four-week observations of 81 flocks were analysed in the model that considers the following fixed and random effects as well as a linear regression.Fixed effects:
1. housing system (HS)
2. house (H) within HS
3. vaccination (V)
4. interaction between HS and V
5. interaction between H and V within HS
6. age (A) in terms of 4-week period
7. interactions between V and A
8. week within four-week period (W)
Linear regression:
9. sequence of the flocks (S)
Random effects:
10. flock (f) within HS, V, and
11. random error
Results
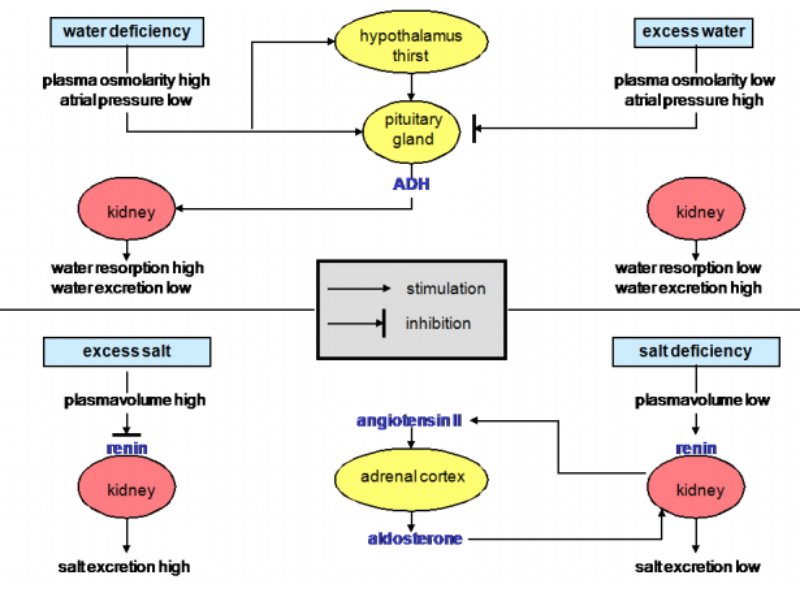
As expected, age (A) effects, represented by the four-week observations, were highly significant, but also the sequence of the flocks influenced both traits, mortality and hen housed rate of lay. Additionally, highly significant were the considered interaction between vaccination and age as well as the week within the period of a four week observation (W(A)) on laying performance. The highest F-value was estimated for the effect age, which therefore has the strongest influence on both traits.Table 2: Test of significance for the considered effects on laying performance and mortality
per hen housed
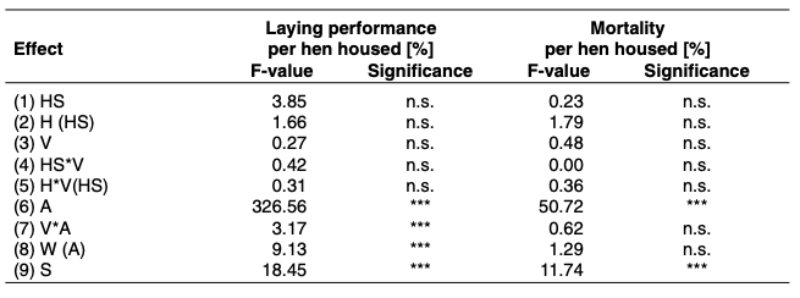
(ADH) is released. This causes the kidneys to retain more water and excrete less. In the event of water excess the release of the hormone ADH and the feeling of thirst are inhibited and thus the amount of water excreted by the kidneys increased. The concentration of sodium in the blood plasma in connection with the renin-angiotensin-aldosterone system is also involved in the regulation of water supply. A reduction in sodium concentration leads to a reduction in the plasma volume. The reninangiotensin-aldosterone system is thus set into operation. It results in an increased resorption of sodium in the kidneys and an increase in plasma volume.
The water taken up is resorbed in the small intestine via osmotic activity. 70% of the body mass of broiler chickens is water (Wilson, 2009). Of this around 50 % is located in the intracellular space and 20 % in the extracellular space which is subdivided into intravasal space and extravasal space (fig. 2). The exchange of water between the fluid spaces is caused by differences in concentration with the aim of achieving osmotic balance.
Figure 2: Fluid spaces
The estimated regression coefficients for the sequence of the flocks are for both traits significant. From flock to flock, rate of lay per hen housed increased by 1.7 %, and mortality decreased by 0.03 % per week. In a full laying year of 52 weeks this is an increase of about 5 eggs per year and 1.5% less mortality
Vaccination
As shown in table 1, two flocks per house were vaccinated with an oil based autogenous vaccine of Lohmann Tierzucht (oil) and a variable number of flocks vaccinated with an autogenous vaccine using aluminium hydroxide (Al(OH)3) as carrier from another company. Although the two types of vaccines were sequentially tested, the Least-Squares estimates for vaccine effects shown in table 2 comparing the two vaccines should be free of the general time trend, which was included as linear regression in the model. Under ideal experimental conditions, the comparisons would have been simultaneous and with untreated controls.
Housing system
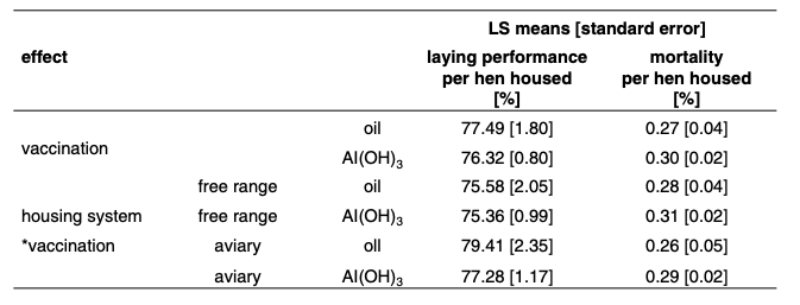
Significant differences between flocks due to the vaccine used can also be seen when looking at the interaction effect between housing system and vaccine type. The Least Squares mean rate of lay was lower when an Al(OH)3 based vaccine (76.3 %) was used than an oil-based vaccine (77.5 %) was inoculated. The same positive tendency was estimated for mortality and can be read in table 3.Weekly mortality is relatively high, with 0.30 % and 0.27 % for the two types of vaccines, but favours the oil-based LTZ vaccine consistently in both types of housing by a margin of .03 % or 1.56 % per year.
The different standard errors of the estimates reflect the fact that more flocks were vaccinated with the Al(OH)3 based vaccine before changing to the LTZ vaccine with oil suspension.
Table 3: LS-Means for hen housed rate of lay and mortality
water
The LS-Means for the two vaccination groups in four-week periods (not shown in detail here) indicate that the main benefit of the oil-based LTZ vaccine is expressed after peak production: insignificant +0.1 % per week from 22-33 weeks of age, but a significant difference of + 1.7 % per week from 34- 69 weeks of age. During the last four-week period, average rate of lay per hen housed was 57.6 % versus 62.0 %. Similar results were found for cumulative mortality, which is included in the trait henhoused rate of lay. Significant differences between housing systems and farms with the same housing system suggest considerable potential for further improvement of farm management irrespective of the housing system and vaccination program. Farm 5 achieved the best performance of all farms, averaging nearly 10 % above the results of farm 2.
Discussion
Consumer demand for safe food and awareness of potential problems due to antibiotic resistance on the one hand and the preference for eggs from non-cage systems on the other hand present a challenge for egg producers and poultry veterinarians to control bacterial infections by vaccination (VAN OIRSCHOT 1994, WHITE et al. 2002). Prevention and control of bacterial infections in laying hens by means of commercial vaccines is often hampered by a lack of efficiency due to the high antigenic variability of the pathogens. Therefore, autogenous vaccines are used instead (BRAGG 2002, WAMBURA 2010). They offer the unique possibility to cover bacterial strains currently responsible for disease on a specific farm. The efficiency of autogenous products is highly influenced by a large number of factors like pathogen detection, typing and selection, regular “update” of the vaccine with the strains currently causing disease as well as infectious pressure in the flock.In this study we tried to elucidate the influence of the adjuvant on the efficiency of autogenous vaccines for laying hens in the field. We therefore conducted a retrospective analysis of performance data provided by five different egg producers with two comparable houses each. In all 10 houses, the last two flocks of layers were vaccinated with an autogenous trivalent vaccine produced by Lohmann Tierzucht and blended using mineral oil as adjuvant (oil), following previous vaccination of several flocks with a comparable vaccine of a competitor using aluminium hydroxide as adjuvant (Al(OH)3).
Weekly rate of lay and mortality was expressed in twelve 4-week periods from 22 to 69 weeks of age for statistical analysis.
To get a reliable estimate of differences due to the vaccine used, we ignored strain of layers (confounded with house within farm and housing system) in the model; other unknown differences due to farm management and nutrition were minimized by using both vaccines on each farm in consecutive flocks.
Ideally, both types of vaccines should have been used simultaneously in the two houses per farm and repeated over two years to get unbiased estimates of the vaccination effect. To estimate the effects of vaccination as free as possible from the general time trend, we included the test year in the model as linear regression over the whole observation period. The regressions of hen-housed rate of lay and weekly mortality on years estimate the combined effects of selection for improved adaptability to non-cage systems and improved environment due to cumulative experience on each farm with a given housing system. The estimated difference of the performance following the oilbased vaccination compared to the alternative vaccine previously used should be free of the general time trend and understood as additional effect on all farms in the last two flocks.
The results found under these conditions indicate that commercial layers vaccinated once during rearing with an autogenous mineral oil based bacterin outperform layers vaccinated twice with an aluminium hydroxide based autogenous vaccine. This implies that an oil-suspension vaccine provides better protection than the aluminium hydroxide based vaccine, which is in accordance with previous studies (MURTHY et al., 2007, BENNECKE 2008, JACOBS et al. 1992). One reason for this result might be the longer duration of the immune response. Duration of immunity is one of the key prerequisites for vaccines used in layers, as laying periods last up to one year after immunisation and a booster vaccination during the laying period is technically more than difficult. Mineral oil adjuvants lead to a prolonged release of antigens, respond directly to the macrophages and thereby display a longer lasting antibody titre level than aluminium hydroxide gel adjuvant vaccines (FUKANOKI et al. 2000).
Several other factors may affect the efficiency of a vaccine like the antigen quantity per dose as well as the production procedure of the antigen. As the vaccine with the aluminium hydroxide as adjuvant was produced by a different producer and their production scheme is not known, our conclusions are limited to the adjuvants, which is the only objective distinguishing feature between the two vaccines.
In conclusion, in this retrospective study the mineral oil based autogenous vaccine produced by Lohmann Tierzucht lead to better performance of commercial layers in comparison to vaccination with an aluminium hydroxide based autogenous vaccine. Furthermore, better performance was achieved with a single immunisation compared to the labour- and cost-intensive double immunisation required by the aluminium hydroxide based vaccine.
Zusammenfassung
Praxisergebnisse mit dem Einsatz autogener Impfstoffe zur Verringerung von E. Coli bei Legehennen in BodenhaltungBei der Aufbereitung von bestandsspezifischen Impfstoffen setzen verschiedene Hersteller unterschiedliche Adjuvantien (Trägerstoffe) zur Verstärkung der Immunantwort ein. In der Regel sind dies verschiedene Mineralöle oder Aluminiumhydroxid (Al(OH)3), deren Einfluss auf die Wirkung des Impfstoffes in diesem Beitrag untersucht wurde. Die hierzu ausgewerteten Felddaten berücksichtigen Leistungsdaten von 81 Legehennenherden, die sich auf fünf Betriebe mit jeweils zwei Häusern – drei Freilandbetriebe und zwei geschlossene Bodenhaltungen mit integrierter Voliere – aufteilen. Die letzten beiden Herden eines Hauses wurden einmalig mit einem bestandsspezifischen Impfstoff der Firma Lohmann Tierzucht geimpft, dessen Adjuvanz eine Ölsuspension ist. In allen anderen 61 Herden wurde den Legehennen hingegen zweimalig ein bestandsspezifischer Impfstoff mit Al(OH)3 als Trägerstoff verabreicht.
Unter Berücksichtigung verschiedener Einflussfaktoren zeigen die einmalig geimpften Legehennen im Vergleich zu den mit Al(OH)3 als Trägerstoff geimpften Hennen eine bessere Legeleistung je Anfangshenne (77,5 % vs. 76,3 %) sowie eine niedrigere Verlustrate (0,27 % vs. 0,30 % pro Woche).
Ähnlich positiv zeigt sich die Wirkung des Öladjuvanz bei einer Betrachtung innerhalb der beiden Haltungssysteme. Eine Verknüpfung der Effekte Betrieb und Haltungssystem macht jedoch deutlich, dass die besseren Leistungsergebnisse in der Bodenhaltung in erster Linie auf den Betriebseffekt zurückzuführen sind. Die Unterschiede in der Legeleistung betragen von Betrieb zu Betrieb bis zu 10 %. Ferner verstärkt die Betriebsentwicklung im Verlauf der neun Beobachtungsjahre die Überlegenheit des Öladjuvanz gegenüber Al(OH)3, durch dessen Einsatz in den letzten beiden Beobachtungsjahren. Der geschätzte Regressionskoeffizient zeigt von Herde zu Herde eine Steigerung in der Legeleistung um 1,7 % und eine Abnahme der Verluste um 0,03 %. Das entspricht einer jährlichen Steigerung der Legeleistung je Anfangshenne um 5 Eier und einer Senkung der Verlustrate um 1,5 % als kombinierter Effekt genetisch verbesserter Anpassung an Bodenhaltungsbedingungen, Erfahrung der Betriebsleiter mit den besonderen Haltungsbedingungen und verbesserter Impftechnik. Die verbesserten Leistungsergebnisse in Kombination mit nur einmaliger und dadurch weniger zeitund kostenaufwendiger Immunisierung sprechen für einen bestandsspezifischen Impfstoff mit Ölsuspension als Adjuvanz.
BENNECKE, K., 2008: Vergleichende Untersuchungen zur protektiven Wirkung einer Streptococcus suis Ganzzell- und einer Subunitvakzine. Diss., Tierärztliche Hochschule Hannover.
BRAGG, R.R., 2002: Isolation of serovar C-3 Haemophilus paragallinarum from Zimbabwe: a further indication of the need for the production of vaccines against infectious coryza containing local isolates of H. paragallinarum. Onderstepoort. J. Vet. Res. 69: 129- 132.
DEGUCHI, K., T. HONDA, K. MATSUO, H. FUJIKAWA, T. IWAMOTO and Y. SAKANOUE, 1998: Influence of inoculation site of combined oil-adjuvanted vaccine on the antibody response in chickens. J. Vet. Med. Sci. 60: 831-835.
FUKANOKI, S., K. MATSUMOTO, H. MORI and R. TAKEDA, 2000: Relation between antigen release and immune response of oil adjuvanted vaccines in chickens. J. Vet. Med. Sci. 62: 571-574.
JACOBS. A.A.C., W. CUENEN, and P.K. STROM, 1992: Efficacy of a trivalent Haemophilus paragallinarum vaccine compared to bivalent vaccines. Vet. Microbiol. 32: 43-49.
MAZAHERI, A., H.C. PHILIPP, H. BONSACK AND M. VOSS, 2006: Investigations of the vertical transmission of Erysipelothrix rhusiopathiae in laying hens. Avian Diseases 50(2): 306-308.
MOREIN, M., M.V. ERIKSSON, A. SJOLANDER and K.L. BENGTSSON, 1996: Novel adjuvants and vaccine delivery systems. Vet. Immunol. Immunopathol. 54: 373-384.
MURTHY, A.K., J.P. CHAMBERS, P.A. MEIER, G. ZHONG, B.P. ARULANANDAM, 2007: Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infection and Immunity, 75 (2): 666- 676.
REID, G.G., and P.J. BLACKALL, 1987: Comparison of adjuvants for an inactivated infectious coryza vaccine. Avian Dis. 31: 59-63.
SAS INSTITUTE, 2004: SAS/STAT User’s Guide (Release 8), Cary NC, USA.
SMART N.L., HURNIK D., MACINNES J.I., 1993: An investigation of enzootic Glasser’s disease in a specific-pathogenfree grower-finisher facility using restriction endonuclease analysis. Can Vet J. 34(8): 487-490.
VANDEKERCHOVE, D., P. DE HERDT, H. LAEVENS, F. PASMANS, 2004: Risk factors associated with colibacillosis outbreaks in caged layer flocks. Avian Pathology, 33(3): 337-342.
VAN OIRSCHOT, J.T. 1994: Vaccination in food animal populations. Vaccine 12: 415-418.
WAMBURA P.M., 2010: Preparation and use of autogenous vaccine from Avibacterium paragallinarum (strain Tan 1-05) in layer chickens. Trop. Anim. Health Prod. 42(3): 483-486.
WHITE, D.G., S. ZHAO, S. SIMJEE, D.D. WAGNER and P.F. MCDERMOTT, 2002: Antimicrobial resistance in foodborne pathogens. Microbes Infect. 4: 405-412.
ZANELLA, A., G.L. ALBORALI, M. BARDOTTI, P. CANDOTTI, P.F. GUADAGNINI, P.A. MARTINO AND M. STONFER, 2000: Severe Escherichia coli O111 septicaemia and polyserositis in hens at the start of lay. Avian Pathology, 29(4): 311-317.










