1Albrecht Daniel Thaer-Institute of Agricultural and Horticultural Sciences (ADTI) at Humboldt-Universität zu Berlin (HU)
2Senior researcher at the Institute for Agricultural and Urban Ecological Projects (IASP) at HU and Private Docent at the ADTI

Cissy Riedel studied Agricultural Sciences at the Albrecht Daniel Thaer-Institute of Agricultural and Horticultural Sciences (ADTI) at Humboldt-Universität zu Berlin (HU). Her literature study with the title „A brighter future for poultry –How the use of light during incubation can affect behaviour, health and performance in chickens“, was supervised by Barbara Tzschentke and represents the basis of this review. She is now working at the Ministry of Agriculture, Environment and Climate Protection in Brandenburg, Germany.
Barbara Tzschentke is senior researcher at the Institute for Agricultural and Urban Ecological Projects (IASP) at HU and Private Docent at the ADTI. Her research is focused on the influence of incubation factors on the development of physiological control systems and energy metabolism in birds. Recent research projects focus on short-term temperature training of late-term poultry embryos. The aim of this research is to investigate the effects of temperature stimulation on immediate embryonic development, hatching results and the long-term impact on health, robustness and performance, but also to develop a non-invasive control system for the application of temperature training in the field. Barbara Tzschentke is board member of the German Group of the Worlds Poultry Science Association (WPSA). Since 2003 she was involved in the establishment of the WPSA Working Group 12 (Physiology) and since 2015 she acts as chair person of this group.
To contact the authors: barbara.tzschentke@iasp.hu-berlin.de
ABSTRACT
Commercial chicks are conventionally incubated and hatch under darkness. The perception of light through retinal and extra-retinal physiological mechanisms develop in the early phase of incubation. Light during incubation influence the hormonal and neuronal system of the embryo and can also affect post-hatch performance and behaviour. These effects are not fully understood
Light is a complex stimulus, which comprises wavelength (colour), intensity and the distribution of the scotic phases over the 24 hours cycle. The eggshell modulates spectrum and intensity of light perceived by the embryo. Characteristics of the eggshell may be a major cause for differences of breeds in response to light during incubation.
Increasing application of LED lights in poultry production enhance the study of particular wavelength on embryonic development and function. The present state of knowledge can be summarized as follows:
1 Light during incubation generally accelerates embryonic growth, independently of wavelength and spectral composition. This does not lead to an increase of chick weight at hatch since the embryo hatches earlier when incubated under a light programme.
2 There is no consistent effect of light during incubation on total hatching rate, chick quality and post-hatch performance in layers and broilers.
3 High light intensity has detrimental effects on hatchability even when applied for a few hours daily.
4 Green light seems to stimulate growth of the embryos and the chicks post-hatch through increased levels of Growth Hormones (GH) and Insulin-like Growth Factor (IGF).
5 Light programmes can stimulate the development of muscles, bones and body functions. This can enhance adaptation to post-hatch conditions when applied during the last days of incubation. There is no evidence that light increases general activity and other behaviours.
6 Direct effects of light on feather pecking and cannibalism have not been proved. But it may indirectly reduce this damaging behaviour through reduced fear and stress.
INTRODUCTION
Light during incubation has not been considered as important for the development and liveability of the embryos and the chicks after hatching. Neither the ancient incubation systems in Egypt or China nor the incubators, which have been introduced in Europe in the 18th century provided light during the hatching period (Sykes, 1991).
In the electric incubators, temperature, humidity, ventilation and turning was regulated automatically but light was not provided. Shafey and AL-Mohsen (2002) assumed that the manufacturer of incubators ignored the influence of light on the embryo.
In recently developed hatching systems like the HatchCare system ( Figure 1. , HatchTech, 2019) or the Patio system (van de Ven et al., 2012) the hatching eggs are exposed to light only during the last days of embryonic development in the hatching system.
HatchCare-sytem: hatcher with light, feed and water (by courtesy of HatchTech).

In most common incubation systems, however, the entire incubation process, with the exception of candling during the transfer from the setter to the hatcher, takes place in the dark. This includes a very important «critical period» in the embryonic development of regulatory and sensory systems in the last days before hatching (Tzschentke, 2007; Tzschentke and Halle, 2016). Here, environmental influences or stimuli are important not only for the immediate embryonic development of body functions, but also for the entire life after hatching.
The influence of light on embryonic development has been considered sporadically in the 1970ties. Experiments on this topic have been reported more frequently from 2000 onwards.
The results showed, that the developing embryos respond to light and light might have positive effects on behaviour, health and performance of the chicks.
Light is a complex stimulus which can influence the embryo through duration and distribution of the scotic phases throughout the 24 hours day, wave length (colour) and intensity and manifold interactions between these criteria.
Studies on light in chickens are further complicated due to the difference between human and birds in the macroscopic structure of the retina and finally in the perception of light colour and intensity. This problem is being addressed by Kaemmerling et al. (2018).
The effect of light can be confounded with other factors, such as temperature (Yalcin et al., 2022) and egg shell characteristics (Maurer et al., 2015). There exist no systematic studies on this complex matter in poultry.
Most experiments on the effect of light on chicken embryos compared the current practice of continuous darkness (24D : 0L) with a photic period of 12 hours (12D : 12L) using different sources of light, including natural daylight, traditional incandescent and fluorescent light bulbs and LED light with different bands of wavelength.
A few studies compare continuous darkness with continuous light or varying scotic periods. The present review addresses the following topics:
1. Development of embryonic light perception and lateralisation
2. Effect of colour, intensity and rhythm of light on embryonic development and hatched chicks
3. Effect of different light conditions under artificial incubation on behaviour, health and performance
MATERIAL AND METHODS
A literature search was done using Google Scholar, the search system Primus of the Humboldt- Universität zu Berlin, and the electronic data basis of PubMed, Science Direct and Europe PMC, with the keywords incubation, chicken, health, behaviour, performance, animal welfare, avian eye, embryonic development.
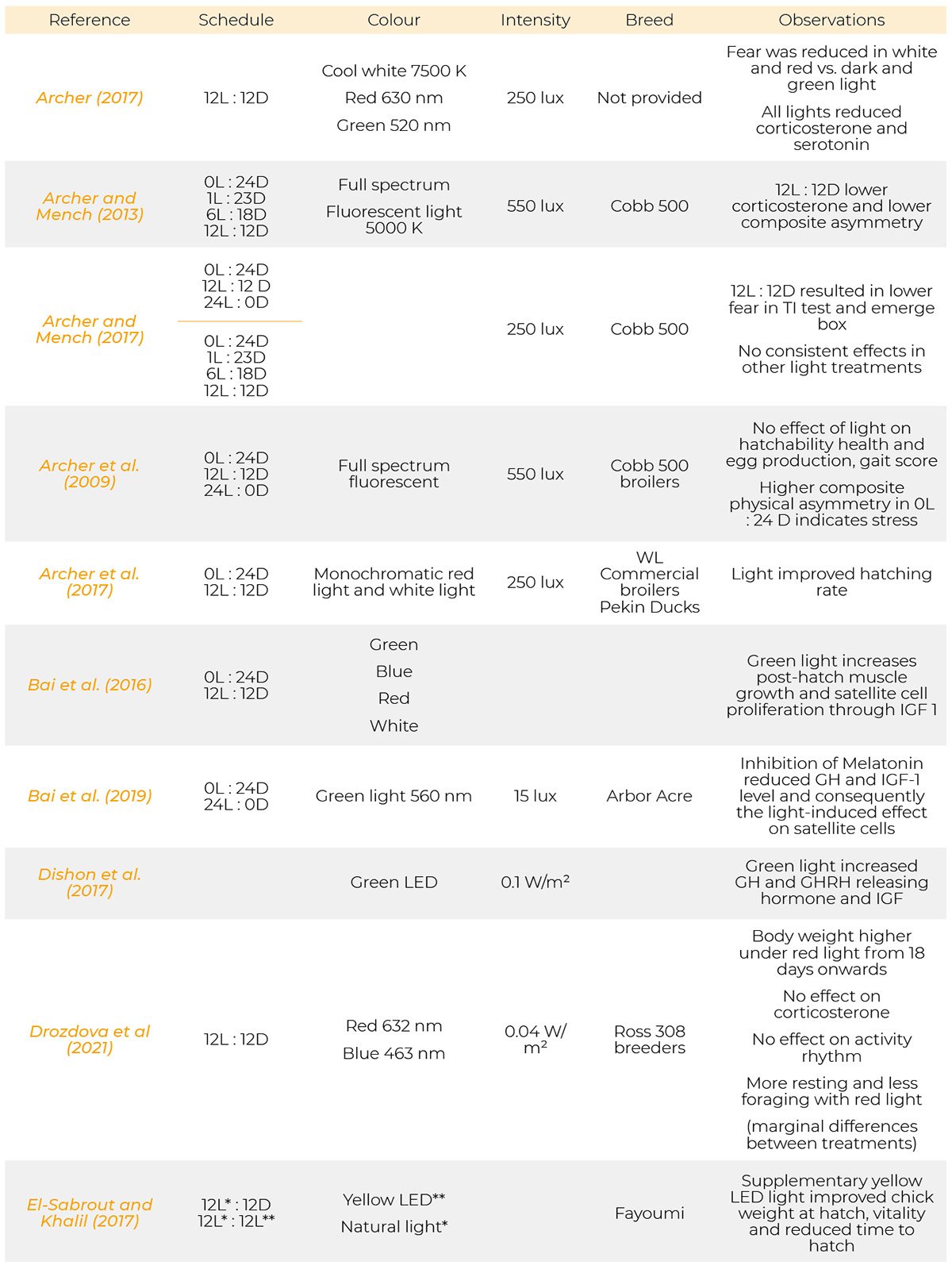
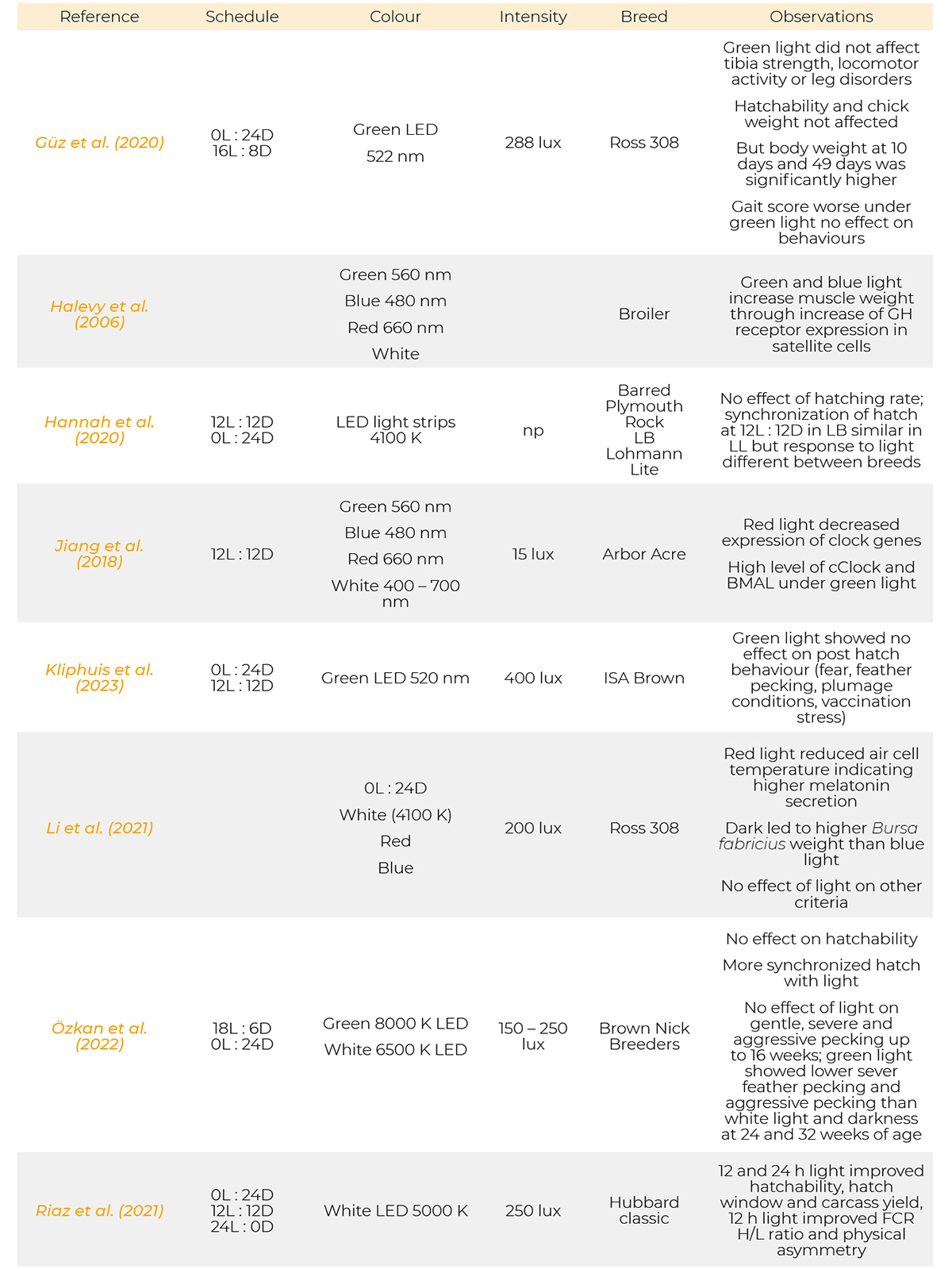
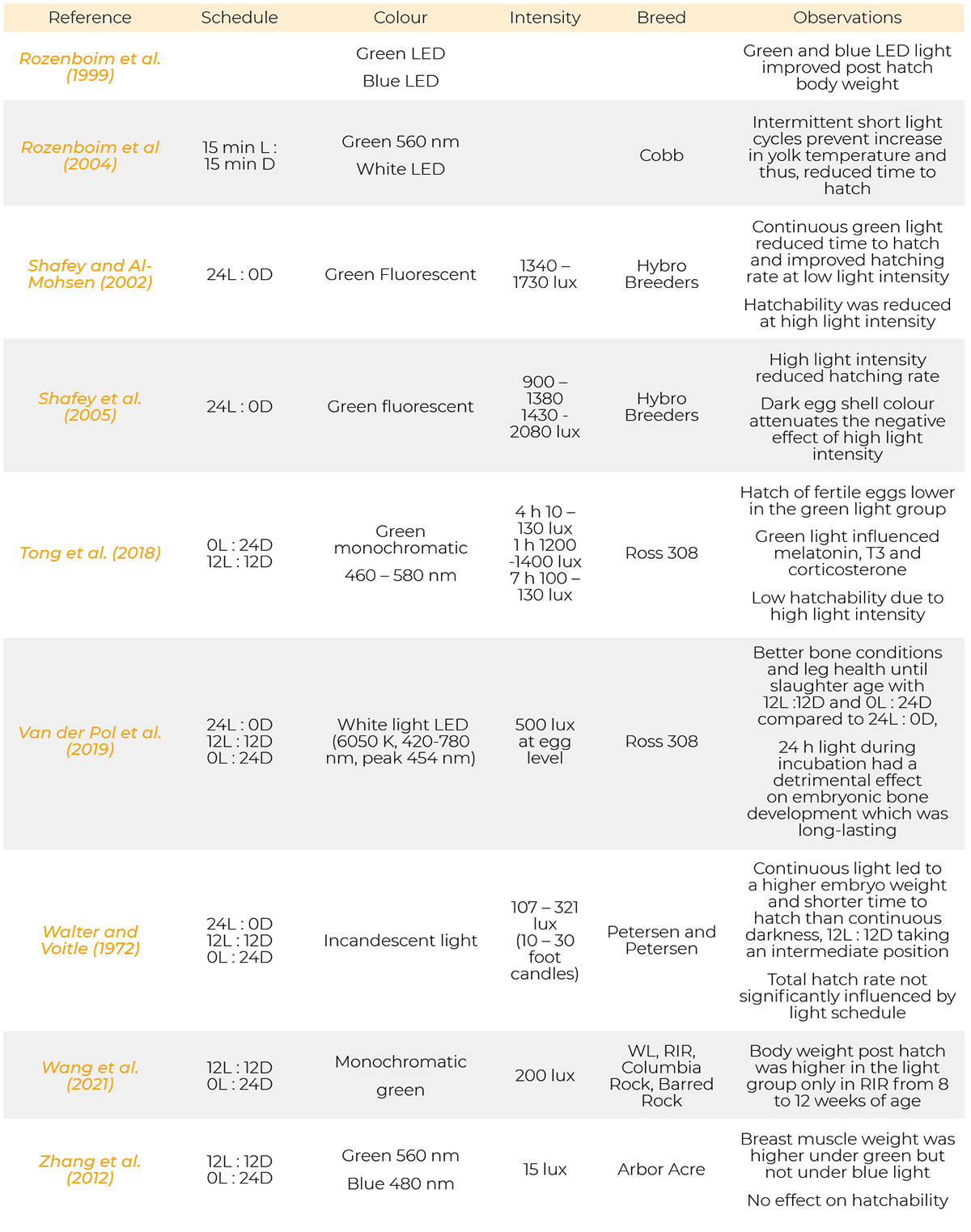
Most references have been published from 1971 to 2023. Only a few are from the period of 1930, 1957 and 1961. Information on the light programs, light colour, light intensity and breeds used in the references cited are provided in table 1.
RESULTS
The development of light perception in the embryo
The visual system of chickens is fully developed before hatching at day 21 (Heaton, 1971, de Haas et al., 2021). Blood vessels, eye ball, lens and the pineal gland develop within the first days of incubation (de Haas et al., 2021). Neural connection between ganglia cells and the optic nerve are also built in the early phase of incubation.
Differentiation of photoreceptors develop later and respond to light stimulation from day 16 onwards. Bruhn and Cepko (1996) found activity in the photoreceptors even at 14 days of incubation.
Full functionality of the eye is reached at day 17 to 18 of incubation (Heaton, 1971).
The production of melatonin in the pineal gland starts at 10 days of incubation. Melatonin is the most important hormone which triggers the response to the light : dark cycle.
According to Schwean-Lardner (2018) the response of the pineal gland to light stimuli becomes functional even at 3 days of incubation. This shows that light can affect the development of the embryo already in the early phase of incubation.
Light perception and lateralisation
Lateralisation is an important event in the function of brain and vision. Lateralisation is the difference in the anatomical and physiological structure of two brain hemispheres which allows the specialisation of neuronal functions in different parts of the brain.
Through the optic chiasm the right hemisphere of the brain is connected to the left eye and vice versa. Consequently, environmental information received by the right and left eye address different brain areas and lead to different behavioural responses.
According to Rogers (2011) and Rogers and Kaplan (2019) the left eye and the right brain hemisphere are controlling behaviour in danger and under stress. They are focused on recognition of social stimuli, maintaining of social hierarchy, and attention to potential predators which lead to fear and stress.
The right eye and the left hemisphere are controlling learned and routine behaviour under stress-free conditions as attention and ability to distinguish objects. Due to the posture of the embryo only the right eye is directed to the egg shell and stimulated by light.
Functional asymmetry of embryonic brains could not be found in chicks hatched in darkness (Chiandetti et al., 2013; Rogers and Kaplan, 2019). Important skills are impaired or not developed. These birds, for instance, cannot distinguish between different objects, show unstable social hierarchies, limited learning ability and higher fear behaviour (Rogers, 2011).
It has been assumed that the light conditions of last three days of incubation are important for the development of lateralisation and the interhemispheric neuronal communication, which is essential for normal development of brain functions and behaviour.
It has been shown, that lateralisation may develop independently of the visual system (Chiandetti et al.,2013) and direct light stimuli on the pineal gland may initiate lateralisation (but not improve interactions between the hemispheres, Roger and Kaplan, 2019) from three days of incubation onwards (Schwean-Lardner, 2018).
The influence of the light schedule vs. continuous darkness
Walter and Voitle (1972) tested three different light programs in a broiler line: continuous light (24L : 0D), continuous darkness (0L : 24D) and a photoperiod of 12L : 12D on time to hatch, development of the embryos and chick weight at hatch, using a 40 Watt incandescent light bulb.
Continuous light led to a higher embryo weight at day 12 and 18 of incubation than continuous darkness.
The 12L : 12D schedule took an intermediate position. Time to hatch was 13 hours shorter under 24L : 0D than 0L : 24D with 12L : 12D showing an intermediate time to hatch.
There was no significant difference in the chick weight at hatch even if the weight was corrected for the weight of the hatching egg.
With the faster embryonic development under 24L : 0D and 12L : 12D the chicks reached the optimum hatch weight earlier than under 0L : 24D.
Total hatch rate was not significantly affected by the light programs. There was a tendency that the hatch rate was higher with 12L : 12D (96%) than with 24L : 0D and 0L : 24 D (94 and 89 % respectively). The navel quality score which ranged from 1 (normal) to 5 (uncomplete retraction of the yolk sac) and chick weight at four weeks of age did not differ in response to the light treatments.
Reduction of hatch time using 12L : 12D with blue LED light from 0 to 9 days of incubation versus continuous darkness throughout the incubation was confirmed in a Barred Plymouth Rock strain (Hannah et al., 2020). But there was no significant difference in hatch time between 0L : 24D and 12L : 12D when the photoperiod was applied from day 17 onwards in two commercial layer strains, Lohmann Brown (LB) and Lohmann Lite (LSL).
Barred Plymouth Rock showed a significantly higher embryo mortality than LB and LSL. The 12L : 12D led to an earlier hatch time when applied from day 0 to day 9 onwards.
But there was no significant effect of the light programs on total percentage of chicks hatched in either line. The hatching window, e.g. the time span from the beginning to the end of hatch, was smaller under photoperiodic light compared to continuous darkness.
The results show, that different breeds respond differently to the light program during incubation. 12L : 12D seems to be more efficient with regard to the acceleration of hatch when applied in an early phase of incubation.
The results have to be considered with care since the effect of light using incandescent light bulbs may be confounded with temperature. Extended periods of light may accelerate embryonic development and shorten the time to hatch through increase of the yolk temperature (Rozenboim et al., 2004). This effect could be eliminated using intermittent light programs with short L : D cycles (15 minutes D : 12 minutes L).
In broiler chickens, the influence of different light durations (0L : 24D, 12L : 12D and 24L : 0D) throughout incubation on leg health at hatching and during the growth period until day 35 post-hatch were tested (van der Pol, 2019).
The results show that 24 hours of light during incubation is detrimental to embryonic leg bone development and long-term leg bone health, and increasing the incidence of tibial dyschondroplasia during the growth phase compared to 24 hours of darkness or a 12 L : 12 D light regime, which long-lasting could stimulate leg health.
Light colour
The use of LED light is developing rapidly in poultry production. LED light not only reduces energy costs but also provides the opportunity to supply light of defined wavelength. It is also suggested that radiation of LED light does not increase the yolk temperature.
El-Shabrout and Khalil (2017) tested 12L : 12D regimes using natural light and yellow LED light in a Fayoumi layer strain. LED illumination did not influence total hatchability, but reduced the time of hatching, increased the chick weight and improved chick quality with regard to activity, navel quality, and leg strength (weakness to stand).
Li et al (2021a) tested white, blue and red light (200 lux) in a 12L : 12 D schedule versus 0L : 24D. There was no significant effect on hatching time, embryonic growth and chick quality.
Archer et al. (2017) compared a light schedule during incubation of 12L : 12D with complete darkness in a White Leghorn layer line, a commercial broiler line and Pekin ducks. Light was provided by a combination of red and white LED lamps with maximum power between 610 and 660 nm wavelength and intensity of 250 lux. Hatch of fertile eggs was significantly better and the incidence of unhealed navels was lower under the 12L : 12D schedule as compared to 0L : 24D.
Hatching in 0L : 24D increased the weight of ducklings significantly but not the weight of layer and broiler chicks. The improvement of the hatching rate through the combination of white and red light was mainly due to a reduced mortality during the first phase of hatching. The authors did not find a similar effect in earlier studies, when only white light or monochromatic red light was used.
Wang et al. (2021) hatched eggs of four different layer breeder stains: White Leghorn, Rhode Island Red, Columbia Rock and Barred Rock under a 12L : 12D monochromatic green light or continuous darkness up to day 18. In all strains green light increased embryonic development and reduced hatch time.
Similar results have been reported by Rozenboim et al. (2013) using green LED light in broiler embryos. The authors suggested that green light penetrates the egg shell und influences the development of muscle cells through retinal and extra-retinal receptors.
Continuous fluorescent green light (24L : 0D) with intensity of 1340 – 1730 lux applied from day 1 to 18 reduced the hatching time by 24 hours, the chick weight by 2 % and improved the overall hatchability in two of 3 consecutive hatches (Shafey and Al-Mohsen, 2002). Hatchability was however reduced by the light treatment in the third hatch.
Continuous fluorescent green light (24L : 0D) with intensity of 1340 – 1730 lux applied from day 1 to 18 reduced the hatching time by 24 hours, the chick weight by 2 % and improved the overall hatchability in two of 3 consecutive hatches (Shafey and Al-Mohsen, 2002). Hatchability was however reduced by the light treatment in the third hatch.
Green light stimulated embryo growth and post-hatch body weight development (Zhang et al., 2012) and breast muscle percentage (Zhang et al., 2016) in a broiler line up to 42 days of age. This effect was mediated through stimulation of Growth Hormone (GH) and Insulin-like Growth Factors (IGF).
Increased post-hatch body weight development in response to green and blue LED light was also reported by Rozenboim (1999; 2013). Green and blue LED light during hatch increased the expression of PBX7 (Paired Box 7) and myogenin which represent important transcription factors for the differentiation of muscle cells (Halevy et al., 2006).
The positive effect short phases of green LED light (15minL : 15minD vs. 0L : 24D) on GH and IGF concentration in the chicken embryo has been confirmed by Dishon et al. (2017). There was, however, no effect of the light treatment on embryo weight, breast muscle weight and liver weight.
Dishon et al. (2021) applied the same light treatment at various phases of incubation. Starting the illumination at day 18 of hatch gave the same results as at any earlier stage of development.
Bai et al. (2019) suggested that the effect of green light on GH and IGF 1 is triggered by melatonin. Blocking pituitary melatonin suppressed the effect of green light on muscle development.
The positive effect of green light on post-hatch body weight has been shown to depend on the genetic line. Wang et al. (2020) tested monochromatic green light (12L : 12 D; 200 lux versus 0L : 24D) on four different breeds. Increased body weight from 8 to 12 weeks of age was found in Rhode Island Red but not in White Leghorn, Columbia Rock and Barred Rock.
Rozenboim et al. (2023) suggested that in broilers the last days of incubation might be a critical period for photo stimulation by green light because of the observed long-term increase in the activity of the somatotropic axis and elevation of post-hatch growth, which could be of high practical relevance.
It would be even more important to use the «right» light in this critical development phase, which is related to the specifics of the bird’s eye and which should, for example, also contain an appropriate UV percentage. Since the body functions throughout life are programmed in this phase, there is a risk of developing persistent false colour vision, which in turn can lead to increased stress.
Light during incubation on behaviour post hatch
Previous studies have shown, that light during incubation elicit various physiological changes which are known to influence the behaviour of chickens. The effect of 12L : 12D schedules under green, red and white light was tested in broilers with regard to fear criteria, such as latency to right in a Tonic Immobility Test (TI) and vocalisation in an Isolation Tests (IT) (Archer, 2017).
Continuous darkness 0L : 24D was used as control. Chicks hatched under 0L : 24D expressed more vocalisation in the IT test and showed a longer latency to right in the TI test than chicks incubated under white and red light. Both criteria are indicators of increased fear. There was no significant difference between 0L : 24D and 12L : 12D under white light.
Similar results have been found by Archer and Mench (2017) using an Emergence Test and a Human Approach Test (Latency to emerge from a box was shorter and duration to stay nearby an unknown human longer in chicks incubated under a 12L : 12D regime as compared to 0L : 24D). Both criteria indicate lower fear of light incubated chicks (see Cavero et al. (2021).
The reduced fear in response to the light treatment can be explained by reduced plasma and serotonin concentration.
Wispels (2017) performed a TI test in chicks hatched under 0L : 24D, 12L : 12D and 24L : 0D. Chicks incubated under 12L : 12D showed a shorter latency to right than chicks under 0L : 24D and 24L : 0D.
The lower fear under the 12L : 12D regime was explained by lower sensivity to stress in the presence of a circadian rhythm. Lower stress in response to 12L : 12D white LED light expressed as low H/L ratio in broilers have been reported by Riaz et al. (2021).
There was no difference in this criterion between 0L : 24D and 24L : 0D. Since continuous light and continuous darkness prevent the development of a circadian rhythm this effect may have affected the stress response in the experiment of Riaz et al. (2021). Archer et al. (2009) studied general activity and feeding behaviour in broiler chicks using white light 12L : 12D, 24L : 0D and 0L : 24D as control.
There was no effect of the light schedules on general activity. Feeding behaviour of chicks hatched under both light regimes was higher during the first 2 hours of light-on compared with 0L : 24D. This may be the result of an entrained circadian rhythm during incubation.
Feed intake was not affected by the treatments as the lower feeding activity after light-on was compensated by higher feeding activity in consequent phases.
Drozdova et al. (2021) observed active (walking, foraging, fighting, wing flapping) and inactive behaviour (resting, standing, pecking, dustbathing) in chicks hatched under red and blue light. The chicks hatched under red light exhibited more passive behaviour while the chicks hatched under blue light more active behaviour.
The difference is explained by the influence of red and blue light on the pineal melatonin rhythm. The maximum of this hormone was higher in embryos hatched under white and red light than under blue light and may lead to more inactive behaviour post- hatch.
Güz et al. (2020) studied walking ability and other behaviours, such as eating, resting, forging, comfort behaviour and dustbathing, of chicks that had been hatched under green light (LED) and 0L : 24D.
Gait score was worse in chicks incubated under green light. This may be related to a slightly higher growth rate. There was no significant effect of the light treatment on the other behaviours.
Lacking effects of hatching under green LED light (12L : 12D; 520 nm; 500 lux) on fear, feather pecking and vaccination stress in ISA Brown pullets have recently confirmed by Kliphuis et al. (2023).
Light intensity
Broiler breeder eggs hatched under continuous light (fluorescent, 1340 – 1730 lux) from day 1 to 18 reduced hatch time (by 24 h) and increased the weight gain of the embryos and hatchability (Shafey and Al-Mohsen, 2002). The chick weight was slightly reduced.
This effect has been attributed to the shorter time to hatch. Using the same light source and schedule under an increased light intensity (1430 – 2080 lux) showed negative effects on embryonic mortality and hatching rate as compared to darkness in birds with light and medium brown egg shell colour but not in dark brown egg shell colour (Shafey et al., 2005).
Dark brown egg shells obviously attenuate the negative effect of high light intensity. Tong et al. (2018) compared a 12L : 12D green monochromatic light schedule with a 0L : 24D as control. One hour of 1200 to 1500 lux was inserted in the light phase of 100 – 130 lux.
The high light intensity phase was provided in order to imitate the light condition under natural hatching. When the mother hen leaves the nest for a short period for eating and drinking the eggs are exposed to high intensity of natural light.
The relatively short phase of high light intensity led to a reduction of hatchability. Information on the effect of light during incubation on feather pecking is scarce. Riedstra and Groothuis (2005) reported higher gentle feather pecking in White Leghorn hatched under light conditions during the last week of incubation.
Although gentle feather pecking does not damage skin and feather cover, its occurrence represents a risk for the development of severe damaging pecking (Rogers and Kaplan, 2019; de Haas et al., 2021). Therefore, Riedstra and Groothuis (2005) recommended not to use light in the last three days of incubation. These results have to be considered with care as the light intensity in the experiment was high (250 – 1000 lux).
Using lower light intensity, Gnan et al. (2005) did not find a difference in feather pecking between pullets which have been incubated under light or dark conditions. There exist relationships between feather pecking and fear and stress (Grams et al, 2015, Archer, 2017; Archer and Mench, 2017).
Since incubation under light conditions reduces fear, it can be speculated that light influences feather pecking indirectly through reduced fear and stress. Özkan et al. (2022) hatched Brown Nick breeder chicks under a 18L : 6D schedule using white 6500K LED and green 8000K LED with a light intensity between 150 to 250 lux.
Gentle feather pecking, severe feather pecking and aggressive pecking were observed at 16, 24 and 32 weeks of age. Different physiological criteria related to stress (corticotropin releasing hormone, CRH) and serotonin (5-HTT), which are considered to influence feather pecking, were assessed on the brain level. There was no consistent effect of the light treatment on the behavioural and physiological traits over the three observation periods.
But there was a tendency for green light of lower intensity than in the experiments of Riedstra and Groothuis (2005) to reduce severe feather pecking and aggressive pecking and to increase 5-HTT levels at 40 weeks of age.
It can be assumed that the direct effects of light on embryonic development and the long-term effects on postnatal development are more positive when a lower light intensity reaches the embryo, as these conditions correspond better to the natural incubation conditions of chickens in a more dimmed environment.
CONCLUSIONS
Although the results of studies on the influence of light during incubation on embryonic development, hatching performance and post-hatching physiology, behaviour, health and primary performance considered in this review are not uniform – ultimately also due to the mostly non-comparable experimental conditions – light application particularly during the last days of incubation and in accordance with the requirements of the chicken’s eye can have a stimulating effect on embryonic and post-hatching development and environmental adaptability.
Furthermore, light application in a critical incubation period as the last days before hatching is obviously essential for the development of certain brain functions that are crucial for the development of different behaviours, cognitive abilities and stress tolerance.
Improved stress tolerance, which could possibly also reduce health problems and behavioural disorders, would be an important welfare aspect. Further systematic studies in this field under comparable experimental conditions that meet the requirements of the chicken’s eye and the specific conditions during incubation are necessary.
Findings from the natural breeding of domestic and wild chickens could make a major contribution to this.
Summary of light schedules, light sources, light intensity, breeds and results of the references.
References
Archer, G. S.; Shivaprasad, H. L.; Mench, J. A. (2009): Effect of providing light during incubation on the health, productivity, and behavior of broiler chickens. Poultry Science 88 (1), 29–37. DOI: 10.3382/ps.2008-00221.Archer, G. S.; Mench, J. A. (2013): The effects of light stimulation during incubation on indicators of stress susceptibility in broilers. Poultry Science 92 (12), 3103–3108. DOI: 10.3382/ps.2013-03434.
Archer, G. S. (2017): Exposing broiler eggs to green, red and white light during incubation. Animal: an international journal of animal bioscience 11 (7), S. 1203–1209. DOI: 10.1017/S1751731117000143.
Archer, G. S.; Jeffrey, D.; Tucker, Z. (2017): Effect of the combination of white and red LED lighting during incubation on layer, broiler, and Pekin duck hatchability. Poultry Science 96 (8), S. 2670–2675. DOI: 10.3382/ps/pex040.
Archer, G. S.; Mench, J. A. (2017): Exposing avian embryos to light affects post-hatch anti-predator fear responses. Applied Animal Behaviour Science 186, S. 80–84. DOI: 10.1016/j.applanim.2016.10.014.
Bai, Xinjie; Wang, Yao; Wang, Zixu; Cao, Jing; Dong, Yulan; Chen, Yaoxing (2016): In ovo exposure to monochromatic lights affect posthatch muscle growth and satellite cell proliferation of chicks: role of IGF-1. In: Growth factors (Chur, Switzerland) 34 (3-4), S. 107–118. DOI: 10.1080/08977194.2016.1199553.
Bai, Xinjie; Cao, Jing; Dong, Yulan; Wang, Zixu; Chen, Yaoxing (2019): Melatonin mediates monochromatic green light-induced satellite cell proliferation and muscle growth in chick embryo. In: PloS one 14 (5), e0216392. DOI: 10.1371/journal.pone.0216392.
Bruhn, S. L.; Cepko, C. L. (1996): Development of the pattern of photoreceptors in the chick retina. The Journal of neuroscience: the official journal of the Society for Neuroscience 16 (4), S. 1430–1439. DOI: 10.1523/JNEUROSCI.16-04-01430.1996.
Chiandetti, C.; Galliussi, J.; Andrew, R.; Vallortigara, G. (2013): Early-light embryonic stimulation suggests a second route, via gene activation, to cerebral lateralization in vertebrates. Scientific reports 3, S. 2701. DOI: 10.1038/srep02701.
Dishon, L.; Avital-Cohen, N.; Malamud, D.; Heiblum, R.; Druyan, S.; Porter, T. E. et al. (2017): In-ovo monochromatic green light photostimulation enhances embryonic somatotropic axis activity. Poultry Science 96 (6), S. 1884–1890. DOI: 10.3382/ps/pew489.
Dishon, L.; Avital-Cohen, N.; Zaguri, S.; Bartman, J.; Heiblum, R.; Druyan, S. et al. (2021): In ovo green light photostimulation during the late incubation stage affects somatotropic axis activity. Poultry Science 100 (2), S. 467–473. DOI: 10.1016/j.psj.2020.10.031.
Drozdova, A.; Okuliarova, M.; Zeman, M. (2019): The effect of different wavelengths of light during incubation on the development of rhythmic pineal melatonin biosynthesis in chick embryos. Animal: an international journal of animal bioscience 13 (8), S. 1635–1640. DOI: 10.1017/S1751731118003695.
Drozdová, A.; Kaňková, Z.; Bilčík, B.; Zeman, M. (2021): Prenatal effects of red and blue light on physiological and behavioural parameters of broiler chickens. Czech J. Anim. Sci. 66 (10), S. 412–419. DOI: 10.17221/80/2021-CJAS.
El-Sabrout, K.; Khalil, M. (2017): Effect of LED Lighting on Hatchability and Chick Performance of Chicken Eggs. PJZ 49 (6). DOI: 10.17582/journal.pjz/2017.49.6.sc2.
Gnan, R.; Hirt, H.; Zeltner, E.; Heß, J.; Rahmann, G. (2005): Einfluss von Licht im Brutschrank auf das Verhalten von Junghennen. Ende der Nische, Beiträge zur 8. Wissenschaftstagung Ökologischer Landbau. https://orgprints.org/id/eprint/3619/, accessed May 2023.
Güz, B. C.; Molenaar, R.; Jong, I. C. de; Kemp, B.; van Krimpen, M.; van den Brand, H. (2021): Effects of green light emitting diode light during incubation and dietary organic macro and trace minerals during rearing on tibia characteristics of broiler chickens at slaughter age. Poultry Science 100 (2), 707–720. DOI: 10.1016/j.psj.2020.11.042.
Haas, E. N.; Newberry, R. C.; Edgar, J.; Riber, A. B.; Estevez, I.; Ferrante, V. et al. (2021): Prenatal and Early Postnatal Behavioural Programming in Laying Hens, With Possible Implications for the Development of Injurious Pecking. Frontiers in veterinary science 8, S. 678500. DOI: 10.3389/fvets.2021.678500.
Halevy, O.; Piestun, Y.; Rozenboim, I.; Yablonka-Reuveni, Z. (2006): In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. American journal of physiology. Regulatory, integrative and comparative physiology 290 (4), R1062-70. DOI: 10.1152/ajpregu.00378.2005.
Hannah, W. A.; Astatkie, T.; Rathgeber, B. M. (2020): Hatch rate of laying hen strains provided a photoperiod during incubation. Animal: an international journal of animal bioscience 14 (2), S. 353–359. DOI: 10.1017/S1751731119002039. HatchTech (2019). Online available under https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjp2_qauvL9AhUphv0HHcbGDIo4ChAWegQIBxAB&url=https%3A%2F%2Fhatchtech.com%2Fwp-content%2Fuploads%2F2019%2F10%2FHatchTech-HatchCare-brochure-EN.pdf&usg=AOvVaw15BRI8G_hioueL_HZpqDrp, accessed March 2023.
Heaton, M. B. (1971): Ontogeny of vision in the Peking duck (Anas platyrhynchos): the pupillary light reflex as a means for investigating visual onset and development in avian embryos. Developmental psychobiology 4 (4), S. 313–332. DOI: 10.1002/dev.420040405.
Jiang, N.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. (2017): Effect of monochromatic light on circadian rhythmic expression of clock genes in the hypothalamus of chick. Journal of photochemistry and photobiology. Biology 173, 476–484. DOI: 10.1016/j.jphotobiol.2017.06.027.
Kaemmerling, D. (2018): Lighting of Poultry Houses to Meet the Needs of Bird Eyes. Lohmann Information 52 (1), 22-30. https://lohmann-breeders.com/media/2020/08/LOHMANN-INFORMATION_VOL52_2018.pdf
Kliphuis, S.; Manet, M. W. E.; Goerlich, V. C.; Nordquist, R. E.; Vernooij, H.; van den Brand, Henry den et al. (2023): Early-life interventions to prevent feather pecking and reduce fearfulness in laying hens. Poultry Science 102 (8), S. 102801. DOI: 10.1016/j.psj.2023.102801.
Li, X.; Rathgeber, B.; McLean, N.; MacIsaac, J. (2021): Providing colored photoperiodic light stimulation during incubation: 2. Effects on early posthatch growth, immune response, and production performance in broiler chickens. Poultry Science 100 (9), 101328. DOI: 10.1016/j.psj.2021.101328.
Maurer, G.; Portugal, S.J.; Nauber, M.E.; Miksik, I., Russell, D.G.D.; Cassy, P. (2015): First light for avian embryos: eggshell thickness and pigmentation mediate variation in development and UV exposure in wild birds eggs. Functional ecology 29, 209-218. DOI: 10.1111/1365-2435.12314.
Ozkan, S.; Yalçin, S.; Babacanoglu, E.; Kozanoglu, H.; Karadas, F.; Uysal, S. (2012): Photoperiodic lighting (16 hours of light:8 hours of dark) programs during incubation: 1. Effects on growth and circadian physiological traits of embryos and early stress response of broiler chickens. Poultry Science 91 (11), S. 2912–2921. DOI: 10.3382/ps.2012-02426.
Özkan, S.; Yalçın, S.; Bayraktar, Ö. H.; Bilgen, G.; Dayıoğlu, M.; Bolhuis, J. E.; Rodenburg, T. B. (2022): Effects of incubation lighting with green or white light on brown layers: hatching performance, feather pecking and hypothalamic expressions of genes related with photoreception, serotonin, and stress systems. Poultry Science 101 (11), S. 102114. DOI: 10.1016/j.psj.2022.102114.
van der Pol, C.W.; van Roovert- Reijrink, I.A.M.; Maatjens. C.M.; Gussekloo, S.W.S.; Kranenbarg; S.; Wijen, J.; Pieters, R.R.M.; Schipper; H.; Kemp, B.; van den Brand, H. (2019): Light-dark rhythms during incubation of broiler chicken embryos and their effects on embryonic and post-hatch leg bone development. PloS ONE 14(1): e0210886. https://doi.org/10.1371/journal.pone.0210886.
Riaz, Muhammad Faisal; Mahmud, Athar; Hussain, Jibran; Rehman, Abd Ur; Usman, Muhammad; Mehmood, Shahid; Ahmad, Sohail (2021): Impact of light stimulation during incubation on hatching traits and post-hatch performance of commercial broilers. Tropical animal health and production 53 (1), 107. DOI: 10.1007/s11250-020-02492-3.
Riedstra, B.; Groothuis, T.G.G (2004): Prenatal light exposure affects early feather-pecking behaviour in the domestic chick. Animal Behaviour 67 (6), S. 1037–1042. DOI: 10.1016/j.anbehav.2003.10.009.
Rogers, L.J. (2011): The two hemispheres of the avian brain: their differing roles in perceptual processing and the expression of behaviour. J. Ornithol., DOI 10.1007/s10336-011-0769-z.
Rogers; Kaplan (2019): Does Functional Lateralization in Birds Have any Implications for Their Welfare? Symmetry 11 (8), S. 1043. DOI: 10.3390/sym11081043.
Rozenboim, I.; El Halawani, M. E.; Kashash, Y.; Piestun, Y.; Halevy, O. (2013): The effect of monochromatic photostimulation on growth and development of broiler birds. General and comparative endocrinology 190, S. 214–219. DOI: 10.1016/j.ygcen.2013.06.027.
Rozenboim, I.; Biran, I.; Uni, Z.; Robinzon, B.; Halevy, O. (1999): The effect of monochromatic light on broiler growth and development. Poultry Science 78 (1), S. 135–138. DOI: 10.1093/ps/78.1.135.
Rozenboim, I.; Piestun, Y.; Mobarkey, N.; Barak, M.; Hoyzman, A.; Halevy, O. (2004): Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poultry Science 83 (8), S. 1413–1419. DOI: 10.1093/ps/83.8.1413.
Rozenboim, I., Dishon, L., Avital-Cohen, N., Bartman, J. (2023): Understanding the effects of light on embryo and post-hatch chick development. In: N. French (Ed.): Embryo development and hatchery practice in poultry production. Cambridge, UK, Burleigh Dodds Science Publishing Limited. DOI:10.19103/AS.2022.0118.
Schwean-Lardner, K. (2018): The effects of hatchery practices on the welfare of poultry. Joy A. Mench (Hg.): Advances in poultry welfare. Duxford: Woodhead Publishing (Advances in farm animal welfare series), S. 29–48.
Shafey, T. M.; Al-Mohsen, T. H. (2002): Embryonic Growth, Hatching Time and Hatchability Performance of Meat Breeder Eggs Incubated under Continuous Green Light. Asian Australasian Journal of Animal Sciences 15 (12), S. 1702–1707. DOI: 10.5713/ajas.2002.1702.
Shafey, T. M.; Al-Batshan, H. A.; Ghannam, M. M.; Al-Ayed, M. S. (2005): Effect of intensity of eggshell pigment and illuminated incubation on hatchability of brown eggs. British poultry science 46 (2), S. 190–198. DOI: 10.1080/00071660500065789.
Sykes, A. H. (1991): An introduction to the history of incubators. In: Avian Incubation. Poultry Science Symposium No. 22. S. G. Tullet Edt., Butterworth & Co. Kent, U.K., 297-303.
Tong, Q., McGonnell, I.M., Demmers, T. G. M., Roulston, N., Bergoug, H., Romanini, C. E., Verhelst, R., Guinebretière, M., Eterradossi, N., Berckmans, D., Exadaktylos, V. (2018): Effect of a photoperiodic green light programme during incubation on embryo development and hatch process. Animal, 12:4, pp 765–773. doi:10.1017/S1751731117002117.
Tzschentke, B. (2007): Attainment of thermoregulation as affected by environmental factors. Poult. Sci. 86, 1025-1036.
Tzschentke, B., Halle I. (2016): Improvement of robustness and performance in meat-type chicken and ducks by short-term temperature training in the hatcher. LOHMANN Information, Vol. 50(2): 30-35. https://lohmann-breeders.com/media/2020/08/VOL-50-Tzschentke-Improvement-of-robustness.pdf
van de Ven, L. (2012): Effects of hatching time and hatching system on broiler chick development. PhD Thesis, Wageningen, The Netherlands.
Walter, J. H.; Voitle, R. A. (1972): Effects of photoperiod during incubation on embryonic and post-embryonic development of broilers. Poultry Science 51 (4), S. 1122–1126. DOI: 10.3382/ps.0511122.
Wang, Panlin; Sun, Yanyan; Li, Yunlei; Fan, Jing; Zong, Yunhe; Isa, Adamu Mani et al. (2021): Monochromatic green light stimulation during incubation shortened the hatching time via pineal function in White Leghorn eggs. Journal of animal science and biotechnology 12 (1), S. 17. DOI: 10.1186/s40104-020-00539-x.
Yalcin, S., Özkan, S., Shan, T. (2022): Incubation temperature and lighting: effects on embryonic development, post-hatch growth, and adaptive response. Frontiers in Physiology, 13:899977. DOI: 10.3389/fphys.2022.899977.
Zhang, L.; Zhang, H. J.; Qiao, X.; Yue, H. Y.; Wu, S. G.; Yao, J. H.; Qi, G. H. (2012): Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poultry Science 91 (4), S. 1026–1031. DOI: 10.3382/ps.2011-01899.
Zhang, L.; Zhu, X. D.; Wang, X. F.; Li, J. L.; Gao, F.; Zhou, G. H. (2016): Green Light-emitting Diodes Light Stimuli during Incubation Enhances Posthatch Growth without Disrupting Normal Eye Development of Broiler Embryos and Hatchlings. Asian Australasian Journal of Animal Sciences 29 (11), S. 1562–1568. DOI: 10.5713/ajas.15.0976.










