Abstract
The EU-funded Prohealth project aims to improve the health of conventionally farmed poultry and pigs by a wide-ranging programme of research into the causation of so-called “production diseases”. The project consortium includes a broad range of skills and interests, including experts on research on consumer attitudes. Here we review the general background to the project and then consider results in the area of consumer perception, biosecurity assessment and establishment of normal gut flora in young birds. The project web-site includes newsletters and references to other project areas also of interest to poultry producers.
Keywords
egg, egg consumption, microbiology, production disease, microbiome, biosecurity, farm hygiene, hatching egg sanitation
Introduction
In 2012 the EU Commission announced its intention to fund research under Framework Programme 7 on Sustainable animal production: an integrated and multi-factorial Approach.The aim of this was to contribute to our understanding of the multi-factorial dimension (infectious agents, genetics, nutrition, and management factors) of diseases of poultry and pigs “linked to the intensification of production, so-called ‘production diseases’”, and so to help us to arrive at effective control strategies and reduce the impact an animal health and welfare.
I was asked to help a consortium of 22 partners led by the University of Newcastle and one of our first tasks was to define what a ‘production disease’ is (the term had previously been applied mainly to metabolic diseases of ruminants). The definition we proposed was“Diseases which tend to persist in animal production systems and, typically, become more prevalent or severe, in proportion to the potential productivity of the system”.
Our bid was successful and the Prohealth project started in December 2013 and will run until November 2018. Our consortium includes a broad range of partners including academic institutions, breeding companies, and small and medium enterprises relevant to the project objectives.
They are active in 10 different EU member states and one associated country and have, collectively, a good geographic spread and involvement in poultry and pig production.
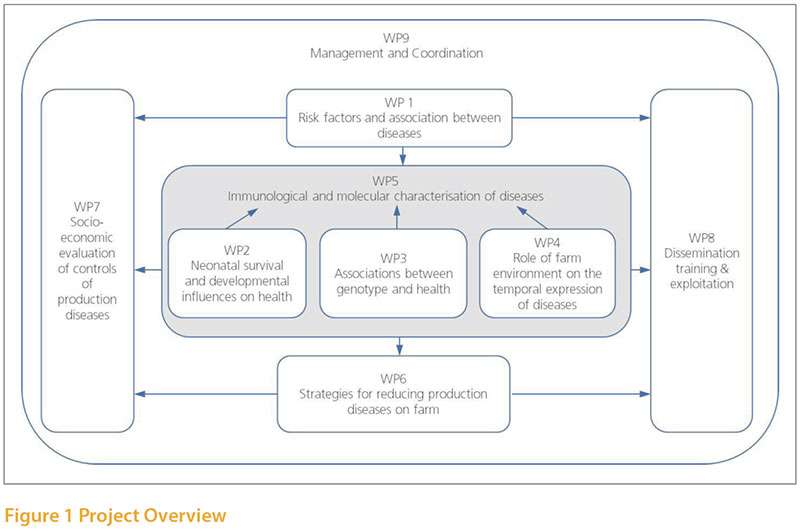 The project has a broad ranging and ambitious
set of objectives to:
The project has a broad ranging and ambitious
set of objectives to:
- Identify the risk factors for production diseases and establish associations between diseases;
- Explore the role of genetic and environmental factors on neonatal survival and in exerting longer-term developmental influences on health;
- Evaluate the effects of genetic selection for productive traits on susceptibility and identify strategies to mitigate these;
- Determine the role of variation in farm environment on the temporal expression of production diseases;
- Characterize the microbio-immunological changes and identify pathological changes at the molecular level which take place during production diseases in order to develop diagnostic tools;
- Synthesize strategies to reduce theimpact of production diseases on a farm and assess the efficacy of improvement strategies in reducing disease prevalence or severity;
- Identify economically viable and socially acceptable ways to control pathologies, with emphasis on animal welfare implications.
The work has been organised in a series of work packages to target a range of issues in the areas outlined below. Much has been achieved on the basic characterisation of production diseases and their risk factors in poultry. Work is currently ongoing on some selected interventions based on these findings and this will be reported in due course. Here we will briefly review some of the completed work which is likely to be relevant to poultry production now and in the future.
Social Science and Economics
Clark et al (2017) have published the findingsof their detailed meta-analysis ofconsumer attitudes to farm animal welfareand identified a gap in relation to thecontrol of production diseases in livestock.They did find quite a lot of variability inattitudes to modern farming but theyalso identified willingness to pay a smallpremium for products associated withimproved disease control and welfare.They found that consumers mostly viewmodern production systems negativelyand tend to voice concerns about naturalnessand humane treatment. Many theyalso tended to be focussed on humanhealth concerns. Most consumers hadlittle knowledge about production diseasesand how they are controlled, thoughthey did tend to refer to antibiotic use. Thesame research group have gone on to carryout a large survey of consumer attitudesin 5 European countries. A range of possibleinterventions were presented and, forlayers, the order of preference found wasas shown in Table 1. Considering that theconsumer claims to have little technicalknowledge about the topic they appearto have chosen quite sensibly those topicswhich might be grouped as ‘good management’as their preferred approach. Giventhe identified low premium that theyare willing to pay, some of these approachesmay not always be economically viable.However, broadly speaking, this worksupports the approach the layer industryhas been taking for many years, of focussingon controlling any egg-associatedhuman health risks and offering productsproduced in different systems. This workalso helps us focus on specific diseasecontrol mechanisms which have consumeracceptance and emphasises that ‘donothing’ is the least favoured approach.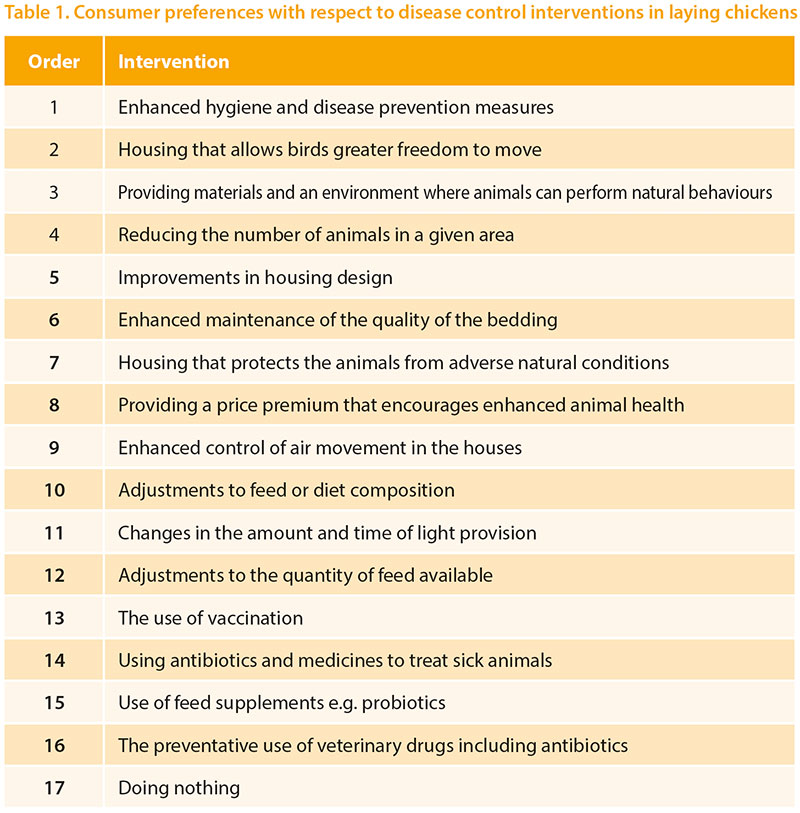
Biosecurity
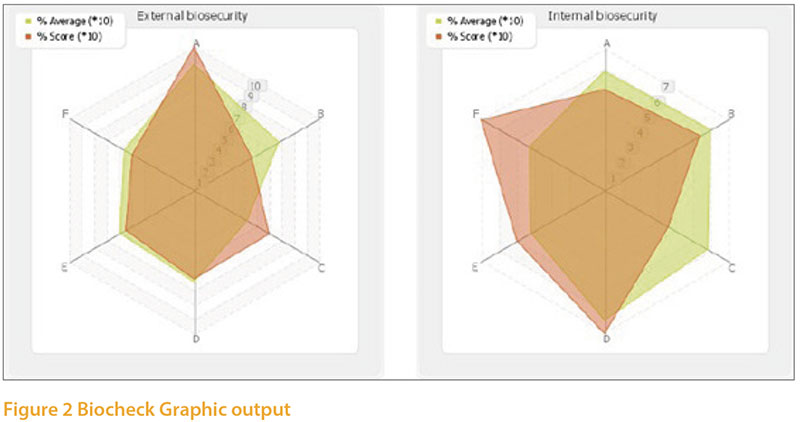
Although biosecurity was not includedas a specific intervention in table 1, it is asignificant component of the number 1choice, “Enhanced hygiene and diseaseprevention measures”. It is, of course, highon our list of priorities currently becausethe unusually high numbers of avian influenzaoutbreaks in both wild birds andpoultry over the past few years. Colleaguesat the University of Ghent have developeddetailed questionnaires to help poultryproducers assess their biosecurity practiceand also applied these independently onfarms of different types. It only takes about20 minutes to carry out the survey and thesystem immediately gives a report withsuggestions for areas of improvement. Separatebiosecurity surveys are freely availablefor broilers and layers :- Layers: https://www.survey.ugent.be/lime/index.php/519199/lang-en
- Broilers: https://www.survey.ugent.be/lime/index.php/981521/lang-en
Farm Hygiene Assessment
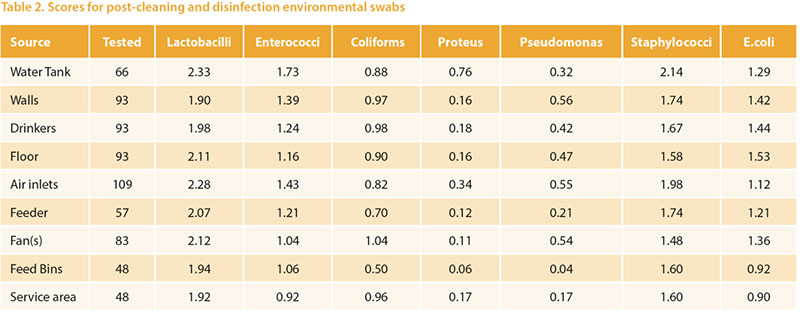
A key aspect of within-farm biosecurity iseffective cleaning and disinfection betweensuccessive flocks in the same farmand building. With increasing regulatoryfocus on the approval of biocides andprotection of the health of workers it isimportant to carefully monitor these processes.Visual assessment is an importantcomponent of this but does need microbiologicalassessment for confirmation.Many assurance schemes require specificmeasures such as absence of Salmonellasp, or Total Viable count on hygiene swabs.Absence of Salmonella is very importantbut tells little about the efficacy of disinfectionagainst other flora, whether pathogenicor beneficial. We developed a simpleprotocol to semi-quantitatively assessthe microbiology of environmental andpost-placement samples. Post-placementsamples were, mainly, boot swabs but alsoincluded dusts, chick-papers etc. Thesewere in varying proportions among the typesof birds examined in accordance withthe types of samples normally submittedfor other purposes. Briefly, the methodinvolves a peptone pre-enrichment (sothat we are focusing on microbes whichfavour body temperatures, and also reducevariability associated with intervalsfrom sampling to testing). The broths arethen diluted and swabbed and streakedon a chromogenic medium developedfor human urinary tract infections (UTIC),and separate media to detect lactobaccili,pseudomonads, and staphylococci. Scoresare logarithmic and take into account dilutionwhere appropriate. The novelty ofthe technique lies in the use of a singlechromogenic agar allowing identificationof major classes of bacteria (Figure 3), andthe use of an enrichment to provide moreconsistent results and favour pathogens.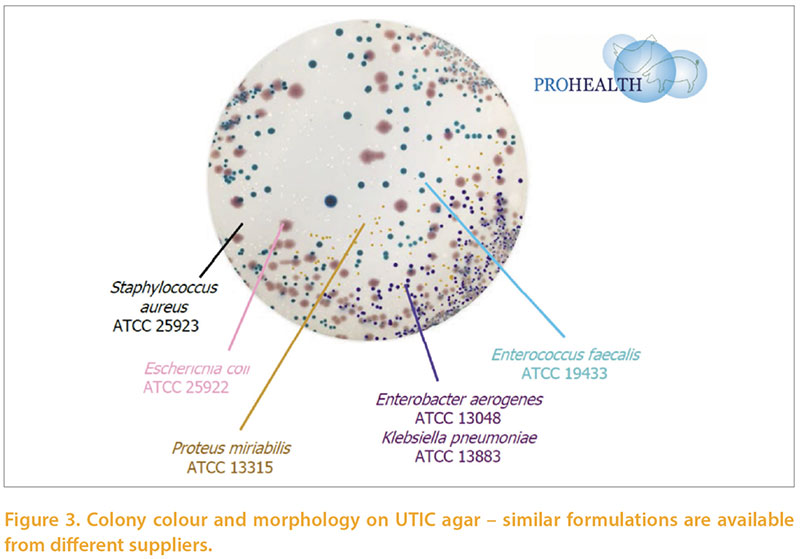 Over 2500 poultry-origin samples were examinedby this method over a 15 monthperiod. The mean scores obtained on postcleaning and disinfecting samples frompullet and layer farms are shown in table2. The rough spectrum of “patho-potential”runs from low on the left to high on theright, and the areas are sorted by the combinedE.coli + Staphylococci score. Themean scores are generally satisfactory, andLactobacilli and enterococci are commonlypresent. Higher scores of pathogens inthis series were more common from watertanks and drinkers, walls and floors.
Over 2500 poultry-origin samples were examinedby this method over a 15 monthperiod. The mean scores obtained on postcleaning and disinfecting samples frompullet and layer farms are shown in table2. The rough spectrum of “patho-potential”runs from low on the left to high on theright, and the areas are sorted by the combinedE.coli + Staphylococci score. Themean scores are generally satisfactory, andLactobacilli and enterococci are commonlypresent. Higher scores of pathogens inthis series were more common from watertanks and drinkers, walls and floors.
This relatively low-tech approach may behelpful in addressing the challenge of controllingproduction-related disease andmaintaining productivity with reduceduse of anti-microbials.We went on to compare pre-placementhygiene and post-placement (boot swab)samples evaluated with the same techniquein over 800 samples from broilerchickens and to evaluate associations withhealth and processing reject parameters(McMullin et al 2017). It was possible toidentify clear differences in microbial profilesbetween samples and sample types.Residual bacterial contamination of farmenvironments showed clear positive andnegative associations with particular flocktraits relevant to production disease. Therewere also significant associations betweenspecific genera in boot swabs samples andrelevant flock traits. The analysis supportsthe need for intervention strategies whichfocus broadly on the relationship betweenpathogen and beneficial organisms alongthe lines of the “Weed/Seed/Feed”-typestrategies which are being used increasinglyin the poultry industry.
Hatching Egg Sanitation
Colleagues in Denmark and Cyprus haverecently published the results of theirstudies on egg sanitation (Olsen et al.2017). This work is particularly interestingbecause there is wish to develop alternativesto formaldehyde in egg fumigation.It demonstrated that repeated treatmentscan substantially reduce microbial loadingof egg surfaces and went on to study the detailed microbiome of egg surfaces. The authors rightly point out that conventionalmicrobiology will only identify culturablebacteria and that 16S RNA sequencing willallow identification of all bacterial speciescolonizing the eggshell of hatching eggs,but it will not distinguish between live ordead bacteria. There may be a role for the use of the enhanced hygiene monitoring protocol inroutine checks of egg sanitation to helpfocus on viable organisms of key pathogenicclasses, supplemented by moleculartechniques where more detail is required.Establishment of normal intestinal flora
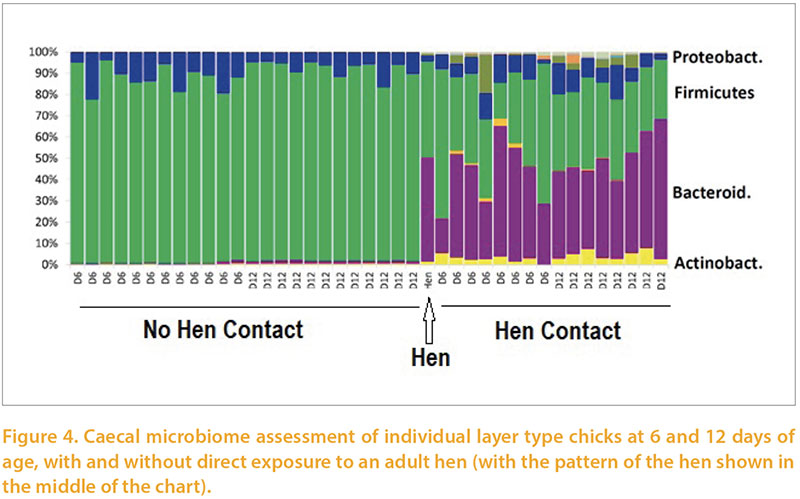
Our colleagues in the Institute of Veterinary Research at Brno provided support to various parts of this project, especially in relation to the composition of intestinal and caecal flora using sequencing of 16s RNA products to classify bacteria regardless of culturability. A nice example of this (Rychlik, 2016) was a series of experiments in which one group of layer chicks were reared from day of age with an adult hen, the other without. Exposure to the hen resulted in a much more diverse and adult-like flora as early as 6 days of age (Figure 4). They have repeated this study on a number of occasions with similar results giving them confidence in the repeatability of this phenomenon. Separate work has demonstrated that establishment of an adult-like flora can take 4-16 weeks in conventionally reared chickens.Rearing commercial pullets along with adult chickens would be likely to have unintended adverse consequences, by, for example, increasing spread of pathogens and heat-stressing the hens. However this work emphasises the potential benefit of using defined or undefined adult flora administration in very early life to help replicate the flora maturation which would occur naturally through exposure to adults. This approach has been widely applied to reduce risk of colonisation with Salmonella sp for over 30 years (Wierup et al, 1987). Recently in-hatchery administration of such products has begun to be widely applied as an alternative to “starter” medication with antimicrobials.
 The same group (Varmuzova et al 2015) have gone on to use a Salmonella Enteritidis challenge model to demonstrate a very obvious activation of inflammatory markers (Figure 5) at day 4 after infection, which was declining by day 14 post infection. The inclusion of 2 plant extracts in the feed markedly reduced this response. However detailed examination of the microbiota showed that the combination of SE challenge and plant extract supplementation resulted in the greatest deviation of flora from the normal pattern. So far this experiment has only conducted once so these findings should be interpreted with caution. While Salmonella Enteritidis is becoming a rare occurrence in most European countries, other pathogens may well interact with the immune system, the microbiota and use of nutritional supplements to destabilise gut flora.
The same group (Varmuzova et al 2015) have gone on to use a Salmonella Enteritidis challenge model to demonstrate a very obvious activation of inflammatory markers (Figure 5) at day 4 after infection, which was declining by day 14 post infection. The inclusion of 2 plant extracts in the feed markedly reduced this response. However detailed examination of the microbiota showed that the combination of SE challenge and plant extract supplementation resulted in the greatest deviation of flora from the normal pattern. So far this experiment has only conducted once so these findings should be interpreted with caution. While Salmonella Enteritidis is becoming a rare occurrence in most European countries, other pathogens may well interact with the immune system, the microbiota and use of nutritional supplements to destabilise gut flora.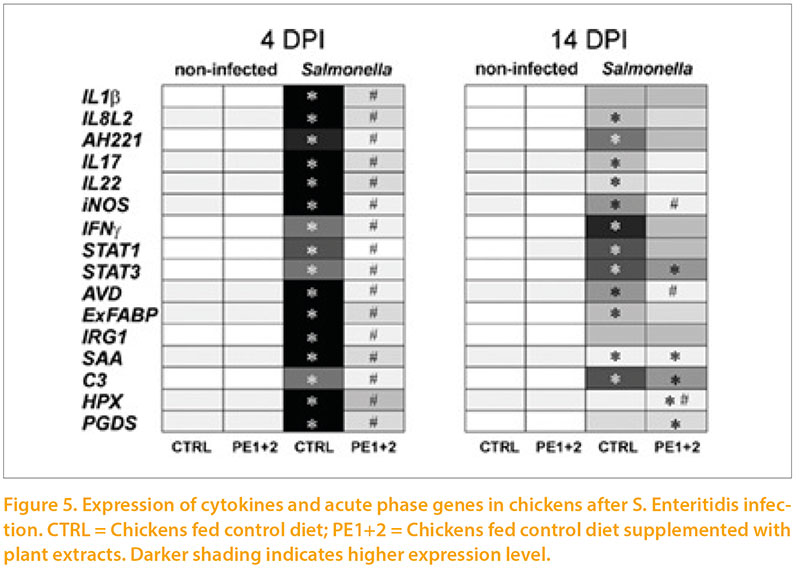
Gene markers and disease
The team at the University of Nottingham veterinary school, has been conducting wide-ranging research on gene-activation associated with disease in poultry, including, in particular coccidiosis and clostridial infection. They are particularly interested in a case of leg weakness in 10 week-old brown-egg layer strain pullets with a histopathological diagnosis of lymphoplasmacytic neuritis. They analysed lymphocyte expression and found that the CD72 gene was up-regulated in these birds. The preliminary findings suggests that while CD8-type cell numbers may be increased, they may not function properly. It is possible that this work will help improve our understanding of so-called B-type Marek’s lesions and/or idiopathic peripheral neuropathy.Other activities
Here we have reviewed only a small proportion of the work carried out under the Prohealth project relevant to poultry. Both the topics discussed and much other useful work carried out in a range of areas will be of interest to the industry. A scientific conference is planned to take place on November 27th/28th 2018 in Ghent, Belgium. Various publications are planned and the web site(http://www.fp7-prohealth.eu) is a good way of following these developments.Finally, readers may find the recently launched “Online Poultry Journal” of interest. This initiative by the consortium provides a consolidated news feed from different sources on topics relevant to poultry and pig health.
This ‘Online Poultry Journal’ may be accessed at: http://www.fp7-prohealth.eu/knowledge-platform/online-poultry-journal/

Clark, B., G B Stewart, L A Panzonea, I Kyriazakisa,
L J Frewer (2017) Citizens, consumers
and farm animal welfare: a metaanalysis
of willingness-to-pay studies.
Food Policy, 68, pp.112-127
Mc Mullin, P., I Nanjiani, S Perez, T van Limbergen, A Ricci (2017) Association between pre-placement and post-placement farm microflora and production disease, productivity and processing rejects in broiler chickens. In Proceedings 20th World Veterinary Poultry Association Congress, Edinburgh. (2017)
Olsen, R, E Kudirkiene, I Thøfner, S Pors, P Karlskov-Mortensen, L Li S, Papasolomontos, C Angastiniotou, J Christensen (2017) Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load Poultry Science, Volume 96, Issue 11, 1 November 2017, Pages 3901–3911
Perez, S, , P Mc Mullin, I Nanjiani (2017) Use of a novel combination of enrichment and semi-quantitative microbiology to improve understanding of pre-placement farm microflora and intestinal flora in poultry. In Proceedings 20th World Veterinary Poultry Association Congress, Edinburgh. (2017)
Rychlik, I (2016) Report on WP.5 Annual Meeting of Prohealth consortium, Athens.
Varmuzova K, ME Matulova, L Gerzova, D Cejkova, D Gardan-Salmon, M Panhéleux, F Robert , F Sisak, H Havlickova, I Rychlik (2015) Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella Enteritidis infection. Poult Sci. 2015 Sep;94(9):2049-58
Wierup, M. M. Wold-Troell, E. Nurmi, M. HÄkkinen (1987) Epidemiological Evaluation of the Salmonella-Controlling Effect of a Nationwide Use of a Competitive Exclusion Culture in Poultry. Poultry Science, Volume 67, Issue 7, 1 July 1988, Pages 1026–1033
Mc Mullin, P., I Nanjiani, S Perez, T van Limbergen, A Ricci (2017) Association between pre-placement and post-placement farm microflora and production disease, productivity and processing rejects in broiler chickens. In Proceedings 20th World Veterinary Poultry Association Congress, Edinburgh. (2017)
Olsen, R, E Kudirkiene, I Thøfner, S Pors, P Karlskov-Mortensen, L Li S, Papasolomontos, C Angastiniotou, J Christensen (2017) Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load Poultry Science, Volume 96, Issue 11, 1 November 2017, Pages 3901–3911
Perez, S, , P Mc Mullin, I Nanjiani (2017) Use of a novel combination of enrichment and semi-quantitative microbiology to improve understanding of pre-placement farm microflora and intestinal flora in poultry. In Proceedings 20th World Veterinary Poultry Association Congress, Edinburgh. (2017)
Rychlik, I (2016) Report on WP.5 Annual Meeting of Prohealth consortium, Athens.
Varmuzova K, ME Matulova, L Gerzova, D Cejkova, D Gardan-Salmon, M Panhéleux, F Robert , F Sisak, H Havlickova, I Rychlik (2015) Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella Enteritidis infection. Poult Sci. 2015 Sep;94(9):2049-58
Wierup, M. M. Wold-Troell, E. Nurmi, M. HÄkkinen (1987) Epidemiological Evaluation of the Salmonella-Controlling Effect of a Nationwide Use of a Competitive Exclusion Culture in Poultry. Poultry Science, Volume 67, Issue 7, 1 July 1988, Pages 1026–1033