Aviadenovirus
Siadenovirus
Atadenovirus (see Table 1).
Natural hosts of EDS virus were originally waterfowl, but historically, the virus was adapted to chickens by contaminated Marek’s vaccines produced in duck-embryo fibroblast cultures in the seventies (EDS 76).
Infections with Fowl Adenoviruses (FAdV)
Only certain serotypes have been associated with diseases in chickens. Within the genus Aviadenovirus 5 species (A to D) have been identified based on the molecular structure, and 12 serotypes based largely on cross-neutralization assays.
As there are major differences in nomenclature of FAdV strains between the US and Europe, the use of the ICTV (International Committee on Taxonomy of Viruses) classification is strongly recommended.
Clinical diseases associated with Fowl Adenovirus
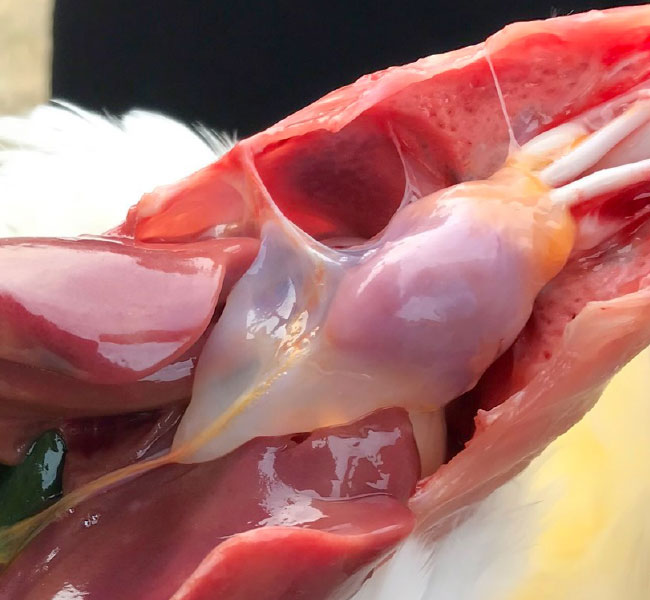
Inclusion Body Hepatitis
- Inclusion Body Hepatitis has first been reported in the early 70th in the US and later in many different countries worldwide (Australia, New Zealand, Europe).
- IBH is mainly caused by FAdV species D and E (serotypes 2, 8 and 12).
- Pathological findings are liver congestion, thymus and bursa atrophy.
- Histopathological inclusion bodies in the liver are predominant.
- The clinical disease is mainly seen in broilers or broiler breeders at the age of 7-18 days of life, indicating vertical transmission of the virus in most cases.
- Mortality may reach 10-40%.
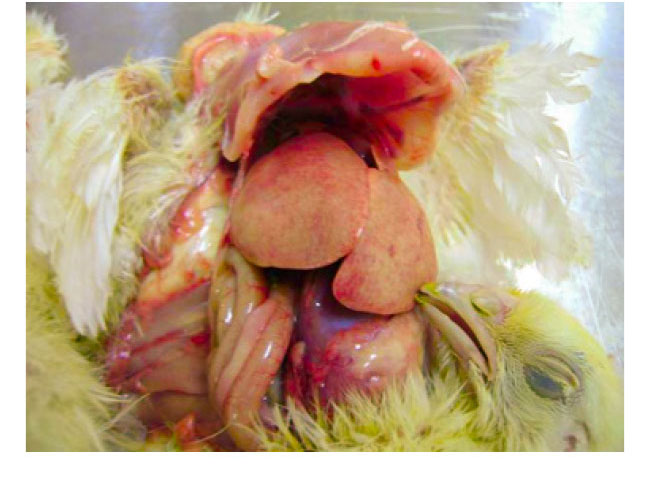
Hydropericardium Syndrome
- Hydropericardium Syndrome was firstly described in Pakistan in 1987 in Angara Goth (“Angara Disease”) and the disease was later diagnosed in India, Iraq, Kuwait and Latin America (Ecuador, Peru, Chile, Mexico). HPS is caused by FAdV Serotype 4 viruses. Pathological findings are hydropericardium, liver congestions with inclusion bodies and thymus atrophy.
- The disease, predominantly seen at the age of 3-5 weeks, is mainly caused by horizontal infections, often associated with severe immunosuppression by other infections like IBD or CAV or by mycotoxins.
- Mortality may reach 12-75% in both broiler and layer type chickens.
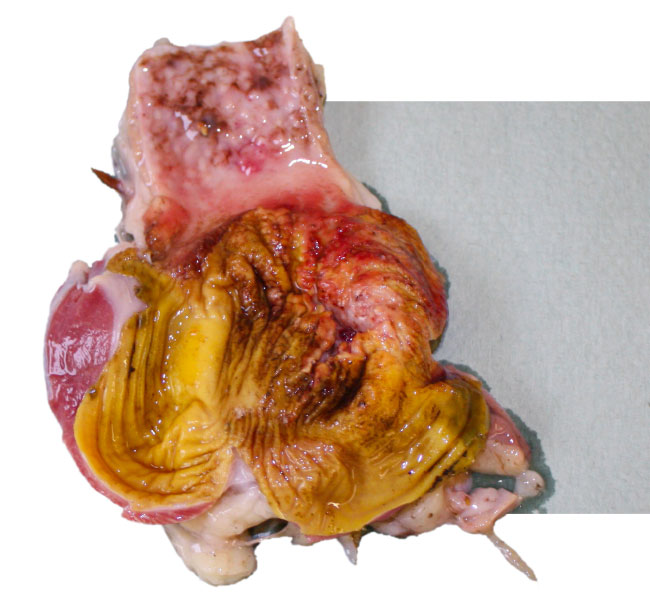
Adenoviral Gizzard Erosions (AGE)
- Gizzard erosions have been associated with Vitamin B6 deficiency or ingestion of histamine, gizzerosine and mycotoxins. Adenoviral Gizzard Erosions (AGE) have been associated with gizzard lesions already in 1981 and are caused mainly by FAdV serotype 1.
- Pathological findings are focal gizzard erosions of the koilin layer, inflammation of the gizzard mucosa and proventriculitis. AGE has been reported in broilers, but occasionally have also been observed in layer type chickens. Clinical disease is rare as the lesions are often only seen during postmortem examination or at the time of slaughter.
- Mortality may vary from 5-15% at the age of 10-21 days, suggesting vertical transmission of the virus in early cases.
Antibody detection by serology and pathogen identification by virology and molecular biology are also essential tools for a correct diagnosis.

Various laboratory tests are available for detection of antibodies against FAdV.
They differ between those detecting antibodies against all serotype’s, like the group-specific Agar Gel Precipitation Test and ELISA’s, and the serotype-specific virus neutralization test (VN).
The Immunofluorescence test (IFT) can detect both antigen and antibodies.
Experimental and commercial ELISA test kits are frequently used for measuring FAdV antibody titers, however these tests can not differentiate antibodies against different FAdV serotypes.
The VN is an essential tool to identify the FAdV serotype in the field if virus isolates cannot be obtained. The VN test is also recommended for monitoring the sero- response after vaccination.
Pathogen identification by virus isolation in chick-embryo liver cells is limited to specific laboratories and uses the identification of Adeno specific cytopathic effects together with fluorescent antibody staining or subsequent PCR testing.
Nowadays molecular biological techniques are commonly used as they are less time consuming and do not require the use of tissue culture techniques. PCR testing followed by DNA sequencing or high-resolution melting (HRM-) curve analysis allows not only the detection but also subtyping of FAdV pathogens in the field.
Suitable samples are:
Prevention of Fowl Adenovirus infections
The strict Biosecurity protocols in breeder flocks during the rearing period might have a negative impact on prevention of Fowl Adenovirus infections: the more isolated the breeder flocks are kept in the rearing period, the more likelihood is an infection with FAdV during the production period, followed by vertical transmission of the virus in a period of 4-6 weeks after exposure until the breeders develop sufficient antibodies to prevent vertical transmission.
Whereas inactivated vaccines are available for the control of Egg Drop Syndrome, no commercially licensed vaccines are available for Fowl Adeno Viruses in most parts of the world.
The aim of vaccination is primarily the prevention of vertical transmission and the protection of day-old chicks by maternal antibodies.
Vaccination of breeder flocks twice in the rearing period at 10-12 and 16-18 weeks of age is recommended.







 兽医
兽医 


