Introduction
Intact boars are rarely used for fattening, because consumers would object to the boar taint, which tends to develop with sexual maturity and renders pork inedible. To eliminate this problem, boars are usually castrated at a young age, a practice which is painful and has been criticized repeatedly as not in line with animal welfare. In 2008, representatives of the German pig farming community, the processing industry and the trade drafted a resolution („Düsseldorfer Erklärung“) to stop castration of piglets without anesthezation. European pig farmers and their union (COPA-COGECA) agreed in December 2010 to terminate surgical castration by 2018. This means that castration of piglets with anesthesia will only be accepted as a transitional step until castration will be completely banned in Europe. However, if intact boars are fattened, negative consumer response to boar taint in pork has to be prevented: by testing carcasses routinely with sufficient speed and accuracy and by reducing the incidence of boar taint at slaughter age. This may be approached in different ways: by genetic selection, nutrition and/or management.Development of boar taint
Boar taint develops under the influence of genetic and non-genetic factors as has been summarized by Bracher-Jakob, 2000. Several studies have shown that the level of skatole and androstenone, the two main components responsible for boar taint, is moderately to highly heritable; the deposition in fat increases with sexual maturity. Non-genetic contributing factors which have been identified are group vs. single pen management and light for androstenone level and nutrition, housing system and hygiene for skatole.Breed differences in boar taint
In order to assess the chances to reduce and eventually eliminate the boar taint by genetic selection, we need to know the relevant population parameters. In table 1 we have summarized results from the literature to show the existing variation between breeds or populations. This may be of special interest for breeding programs which focus on quick response. However, these estimates should not be taken at face value without taking all essential factors into account: age and live weight at the time of testing, management conditions, laboratory techniques applied, and sample size. As pointed out by Haugen (2009), neither are official reference methods available to determine and compare androstenone and skatole levels, nor are all results have been published.The relevance of laboratory techniques has been demonstrated by Harlizius et al. (2008), whose results from different laboratory methods differed by a factor of 2 to 4 for identical samples of backfat. This should be kept in mind; for genetic evaluation, genotypes must always be compared under the same conditions.
The critical level of 0.5 µg androstenone per gram fat, above which most people would notice the boar taint, was exceeded in all but one small sample in table 1.
Typically, more than 40% of boar carcasses would be “off-odor“ according to the androstenone level. Comparing different breeds, Durocs and the early maturing Piétrains tend to have the least desirable taint scores. In the ongoing project called Electronic nose, breeding, entire boar fattening (EN-ZEMA), funded by the German Federal Ministry of Food, Agriculture and Consumer Protection, about 1,000 progeny of Piétrain sires are currently being tested for meat taint in addition to conventional performance criteria.
Currently 800 boars with measurements of this study are available. 38% of the boars exceeded the critical levels of 250 ng skatole and 1,000 ng andostenone per gram backfat; skatol and andostenone
Table 1: Breed differences in androstenone level
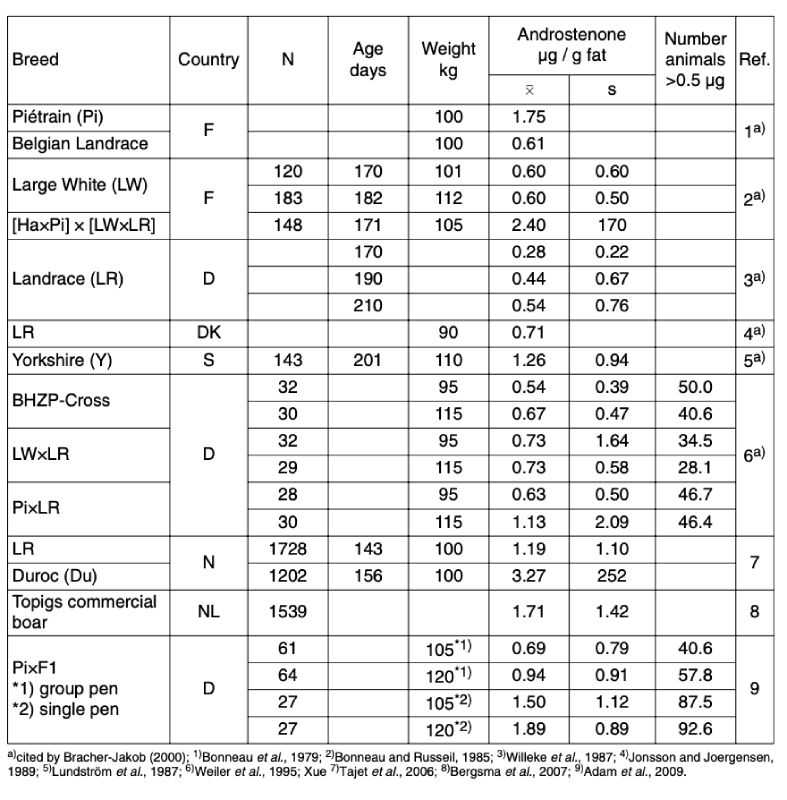
contributing similarly to this percentage. The skatole levels are more likely to be influenced by nongenetic factors than andostenone levels. Nutrition, management, hygiene and the point of sampling (backfat vs. bacon) can influence the skatole level measured and limit the accuracy of genetic evaluation.
Reduction of boar taint by conventional selection
The breeding goals and selection indexes in commercial pig breeding programs include a number of traits defining the efficiency of live weight gain, carcass value and reproductive performance. To predict and achieve the desired genetic progress in specific traits, we need to know the relevant population parameters. As shown in table 2, the androstenone level apparently has a high heritability, and the somewhat lower heritability estimates for skatole and indole levels are also encouraging.Table 2: Heritability estimates for androstenone, skatole and indole level
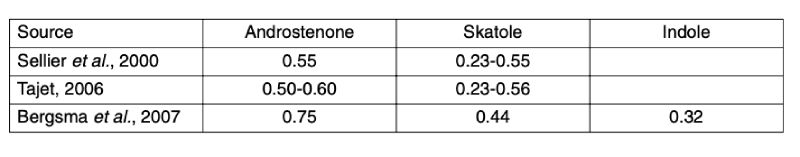
Assuming that the skatole level can be significantly reduced by management and nutrition, genetic approaches may focus on controlling androstenone.
Androstenone is produced in the gonads along with other sexual steroids, androgens and estrogens. Therefore we should be aware of possible antagonistic correlations between androstenone and reproductive performance (Claus, 1993). Published estimates of genetic correlations between andostenone and paternal or maternal reproductive traits are, however, rare.
Bergsma et al. (2007) reported antagonistic correlations between androstenone and paternal fertility in terms of sperm motility (0.32), ejaculate volume (0.18) and livability of sperm (0.11), whereas the correlation with sperm concentration pointed in the desired direction (-0.22). In the same study, antagonistic correlations were found between androstenone levels in backfat probes and maternal reproductive performance in terms of sexual maturity and age at first insemination (-0.24), interval between weaning and subsequent conception (-0.44) and number of stillborn piglets (-0.59).
Willeke (1987) concluded from his analysis that selection for reduced androstenone level would have the undesirable effect of increasing the age at sexual maturity of boars as well as sows. Sellier et al. (2000) tried index selection for lower androstenone level while keeping the size of the bulbourethal gland constant, but failed.
Own study
To answer the question of an assumed commercial breeder who has to determine which selection approach is most promising, we modeled several different scenarios, applying index theory (proportional index) to predict the possible reduction of androstenone levels (Tholen and Frieden 2010). Table 3 shows the traits to be measured. It is assumed that the androstenone level can be measured in live boars from backfat probes obtained by microbiopsyTable 3: Traits to be measured for performance testing boars

Table 4 shows the relative importance and expected economic progress in the traits included in the index as breeding goal, separately for dam and sire lines. The parameter estimates are based on own analysis of German herdbook data and literature (Sellier et al., 2000). For androstenone we assumed a heritability of 0.50.
Table 4: Breeding goals and predicted relative economic progress (in %)
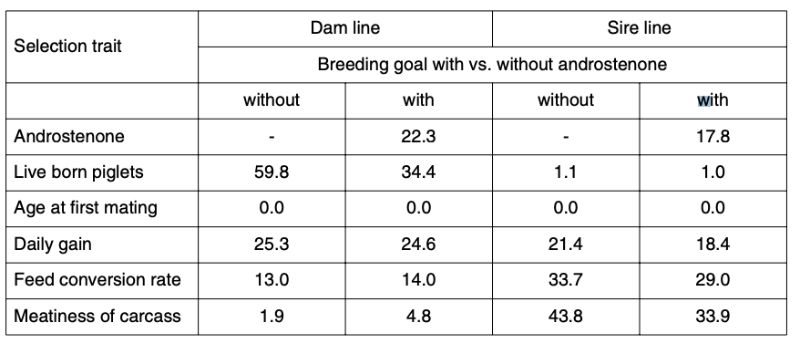
The economic weights (w) differ considerably between the sire and dam lines: the dam lines are mainly selected for number of piglets weaned per year, the sire lines for carcass composition. Change in age at first service was set to zero for all lines. Antagonistic correlations between androstenone level and reproductive performance were assumed to be rg = |0.2|.
The economic weight for androstenone level was determined with the condition that 80% of the progress in conventional traits should be retained. In each generation the best 10% males and 50% females are selected in both lines.
Inclusion of the androstenone level in the index results in significantly less progress in reproductive performance, but slightly more progress in carcass value in the dam line, whereas considerable progress in meatiness is sacrificed in the male line to achieve a reduction in androstenone levels.
Figure 1: Predicted frequency of boars with >1 µg/g androstenone in backfat probes if this
trait is included in a proportional selection index
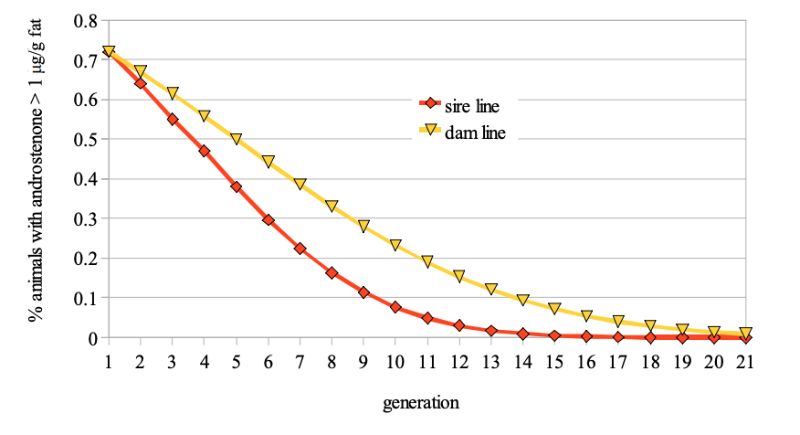
The model calculations suggest that at least 4 to 6 generations, i.e. 8 to 12 years would be required to reduce the frequency of boars with >1 µg/g fat from 20% to 5%, even with optimistic assumptions regarding the antagonistic correlation between fertility and boar taint (fig. 1).
Our estimate of time required for a genetic solution may be compared with the result of Ducro-Steverink (2006) who calculated less than 5 years to reduce the incidence of boar taint from 30% to 10%, assuming a heritability of 0.40 for the androstenone level and ignoring negative changes in reproductive traits.
Reduction of boar taint with molecular genetic methods
Another breeding strategy to reduce boar taint in pork would be to identify the relevant genes with DNA chips. The pig genome has been almost completely sequenced, which offers the possibility to search for DNA markers associated with boar taint.Using genome analysis, the genome of individuals is described in terms of SNP (Single Nucleotide Polymorphism) markers and compared with the phenotypic expression of the relevant trait. Several recent studies in Europe have identified markers for boar taint. In a Dutch project reported by Duijvesteijn et al., (2010), 13.7% of the additive genetic variation in androstenone level could be explained by the five most important SNPs.
As a second step, genomic selection would be applied to identify and select individuals with the desired genotype of low androstenone level without the need for trait recording.
On first sight, genomic selection may seem to offer a quick and easy solution. Before drawing premature conclusions, the results of Grindflek et al. (2010) should be noted who found markers for fertility traits on the same locations of the chromosome as for androstenone level, which is not surprising in view of the described antagonistic effects. Moreover associations between markers and traits are known to be breed specific. In any case, genetic markers have to be identified in each population, with relevant correlations to other traits, before genomic selection is applied in practice.
Discussion and outlook
The intensity of boar taint in carcasses of intact boars can be reduced by selection. This can help the pork industry in gradually reducing the number of carcasses discarded because of boar taint and eventually eliminate the need for castration. To achieve optimal response to selection, standardized procedures for measuring the two main components of boar taint, androstenone and skatole, should be developed. Two current research projects (Anon, 2009a,b) are focused on the development of automated measurement of boar taint for use in slaughter lines of commercial abattoirs as well as on live animals for selection purposes. The eventual goal is to develop techniques for screening live boars for taint score, based on microbiopsy of backfat, saliva or blood samples, which would speed up genetic progress.The rate at which genetic progress can be reached will depend on antagonistic correlations between boar taint and reproductive traits. These genetic correlations have to be determined in relevant commercial male and female lines.
When identified QTLs for boar taint are being used in genomic selection, special attention should be on gene locations which are not known to be negatively correlated with reproductive performance.
Under current economic conditions in Germany it would make sense to screen terminal sires for boar taint before they are widely used for AI. This approach is currently being field tested with the German Piétrain population in the EN-Z-EMA project (Anon, 2009a). In case this approach does not lead to desirable results, testing of boars will be extended to all male and female lines.
Including the reduction of boar taint in the breeding goal will in any case decrease the rate of progress in other traits, which can mean a loss of competitiveness. A breeding organization may expect benefits from a significantly reduced rate of boar taint:
1) if commercial slaughter houses introduce incentives by paying a premium based on the rate of discarding carcasses due to boar taint; and/or
2) if growing intact boars is significantly more economical than growing castrated males in terms of feed conversion ratio and carcass value (Adam, 2009).
With increasing production of pork from intact boars, the processing industry has to expect substantial losses, because pork with boar taint has no market value. Any potential benefit of growing intact boars can only be realized if the frequency of rejected carcasses is substantially reduced below a critical level of 10% or even less. It will take a considerable number of years to find out whether the European pork industry will be successful in eliminating the need for castrating boars as postulated by animal welfare.
Zusammenfassung
Züchterische Möglichkeiten zur Verminderung der Ebergeruchsproblematik bei SchlachtschweinenDie Ferkelkastration in seiner bisherigen Form wird keine Zukunft in der EU haben. Es gibt einige Alternativen, wie z.B. die Ebermast. Hierbei stellt der Ebergeruch, welcher hauptsächlich durch die zwei Komponenten Androstenon und Skatol bestimmt wird, ein Problem dar. Allerdings kann Skatol durch Fütterung, Haltungsform und Hygiene reduziert werden, dagegen wird Androstenon hauptsächlich durch genetische Komponenten beeinflusst. Deshalb ist die züchterische Bearbeitung des Ebergeruchs vielversprechend aufgrund der hohen Erblichkeit. Ein Problem stellt dabei die unerwünschte Beziehung des Ebergeruchs zur maternalen und paternalen Fruchtbarkeit dar, die im Züchtungsprogramm berücksichtigt werden muss. Bei dem derzeitigen Stand wird es zwischen 8 und 12 Jahren dauern, um den Anteil Eber mit über 1000 ng Androstenon je g Fett von 20 auf 5 % zu reduzieren. Eine Verkürzung dieser Zeitspanne könnte die Genomische Selektion bieten. Jedoch wird die Selektion gegen Ebergeruch nur dann erfolgreich sein, wenn eine zuverlässige Technologie zur Verfügung steht, wie z.B. die „elektronische Nase“, die einen mit Ebergeruch behafteten Schlachtkörper am Schlachtband eindeutig identifiziert.

References
Anon., 2009a: Forschungsprojekt “Vermeidung von Ebergeruch durch züchterische Maßnahmen und neuartige messtechnische Erfassung (EN-Z-EMA)” bewilligt. http://www.zds-bonn.de/vermeidung_von_ebergeruch_durch_ zuechterische_mass.html.Anon., 2009b: Verzicht auf Ferkelkastration – QS bringt Forschungsprojekte voran. http://www.q-s.de/ fileamin/QS _Fileadmin/tt_news/091126_PM_ Foerderung_Forschungsprojekte.pdf
Adam, F., C. Schulze-Langenhorst und L. Büttfering, 2009: Düsser Ergebnisse zur Ebermast. Landwirtschaftliches Wochenblatt, 43.
Bergsma, R., E. Knol and H. Feitsma, 2007: Parameters of Al boars and predicted correlated responses of selection against boar taint. Proc. EAAP Meeting, Dublin.
Bracher-Jakob, A., 2000: Jungebermast in Forschung und Praxis. Arbeit im Auftrag der Eidgenössischen Forschungsanstalt für Nutztiere, Posieux.
Bonneau M. et al., 1992: Contributions of fat androstenone and skatole to boar taint: I. Sensory attributes of fat and pork meat. Livestock Prod. Science 32, 63–80.
Claus, R., 1993: Die unendliche Geschichte der Ebermast – Eine historisch-physiologische Analyse. Fleischwirtschaft 73, 449 – 453.
Duijvesteijn, N. et al., 2010: A genome-wide association study on androstenone levels in pigs reveals a cluster of candidate genes on chromosome 6. BMC Genet. 2010 May 20, 11:42.
Grindflek, E, 2010: Revealing genetic relationships between compounds affecting boar taint and reproduction in pigs. J Anim Sci. 2011 Mar;89, 680-92.
Harlizius, B. et al., 2008: Breeding against boar taint. An integrated approach. EAAP meeting, 26-27. March, Girona.
Haugen, J.E., 2009: Detection of Boar Taint. Need for harmonised methods and rapid methods. 55. Intern. Meat Science and Technology Congress, Copenhagen. http://www.icomst2009.dk.kappa.headline.dk
Moe, M. et al., 2009: Association between SNPs within candidate genes and compounds related to boar taint and reproduction. BMC Genet. 2009; 10: 32.
Sellier, P., Le Roy, P., Foilloux, M.N., Gruand. J., Bonneau, M., 2000: Responses to restricted index selection and genetic parameters for fat androstenone level and sexual maturity status of young boars. Livestock Prod. Science 63, 265-274.
Tajet H., Andresen O., Meuwissen T., 2006: Estimation of genetic parameters of boar taint; skatole and androstenone and their correlations with sexual maturation. Prevention of Boar Taint in Pig Production. 19th Symp. Nordic Committee for Veterinary Scientific Cooperation, Gardermoen, Norway, 21-22 November 2005.
Tholen, E. and L. Frieden, 2010: Züchterische Möglichkeiten zur Vermeidung von Ebergeruch. 8. Schweine-Workshop “Neue Herausforderungen für die Schweinezucht”, 23./24.2.2010, Uelzen.
Weiler, U., M. Dehnhard, E. Herbert and R. Claus, 1995: Einfluss von Geschlecht, Genotyp und Mastendgewicht auf die Androstenon- und Skatolkonzentration im Fett von Mastschweinen, 14-32. In: Die Ebermast, Angewandte Wissenschaft Heft 449, Schriftenreihe des Bundesministeriums für Ernährung, Landwirtschaft und Forsten, Landwirtschaftsverlag Münster.
Willeke, H., R. Claus, E. Müller, F. Pirchner and H. Karg, 1987: Selection for high and low level of 5 α- androst-16-en-3- one in boars. I. Direct and correlated response of endocrinological traits. Journal Animal Breeding and Genetics 104, 64-73.
Xue, J. et al., 1996: Breed differences in boar taint: relationship between tissue levels boar taint compounds and sensory analysis of taint. J. Anim. Sci. 74:2170-2177.






