Abstract
In spite of significant improvement in technology and hygienic practices in developed countries at all stages of poultry production
foodborne diseases remain a persistent threat to human and animal health. Beside the current legislations, the main strategy to control
microbial food borne hazards should include Good Animal Husbandry Practices (GAHPs) at the farm level through sound hygienic measures, applied in poultry houses, environment and feed manufacture, as well as reducing colonization with feed additives, competitive
exclusion, treatment or vaccines. Furthermore, hygienic measure should also be considered during transport and slaughtering. In all
cases, surveillance and monitoring programmes must be adapted and followed strictly in aim to allow early intervention. In addition,
the development of antibiotic resistant bacteria will also be a continuous public health hazard.The present paper describes the main
strategy to control food borne infections in poultry, with special attention to European legislations toward safe poultry meat.
Keywords
Poultry, foodborne diseases, salmonella, campylobacter, antibiotic resistance, regulationsCo-author
Hosny El-Adawy, Friedrich-Loeffler-Institute, Institute of Bacterial Infections and Zoonoses, Jena, Germany
Introduction
In spite of significant improvements in technology and hygienic practices at all stages of poultry production in developed countries, accompanied by advanced improvement in public sanitation, foodborne diseases remain a persistent threat to human and animal health. Food borne diseases are still big issues of major concern in those countries. In developing countries, the need to produce sufficient food to meet the requirements of population increases, accompanied by bad economic situations often overshadow the need to ensure safe food products. Regardless of this fact, safe food is a fundamental requirement for all consumers, rich or poor. Food safety is not a discovery of recent times; it is a natural basic instinct of human survival. During human evolution, several approaches were adopted to achieve safety of food. One of the most famous approaches was practiced by several kings, which would employed official and well trusted „tasters“ that served as food safety sentinels for the kings and royal family members. Food safety and quality of food are currently big issues of major concern. Many reports during recent years have shown that Salmonella and Campylobacter spp. are the most common causes of human foodborne bacterial diseases linked to poultry (Figure 1). In some areas also verotoxin producing Escherichia coli 0157:H7 (VTEC), Listeria and Yersinia have surfaced as additional foodborne pathogens causing human illness. Several other toxicogenic bacterial pathogens, such as Staphylococcus aureus, Clostridium perfringens, Clostridium botulinum and Bacillus cereus can also enter the human food chain via contaminated poultry carcasses. In addition, the development of antibiotic resistance in bacteria, which are common in both animals and humans, such as Methicillin Resistant Staphylococcus aureus (MRSA) and Extended-spectrum beta-lactamase (ESBL) bacteria, are also an emerging public health hazard.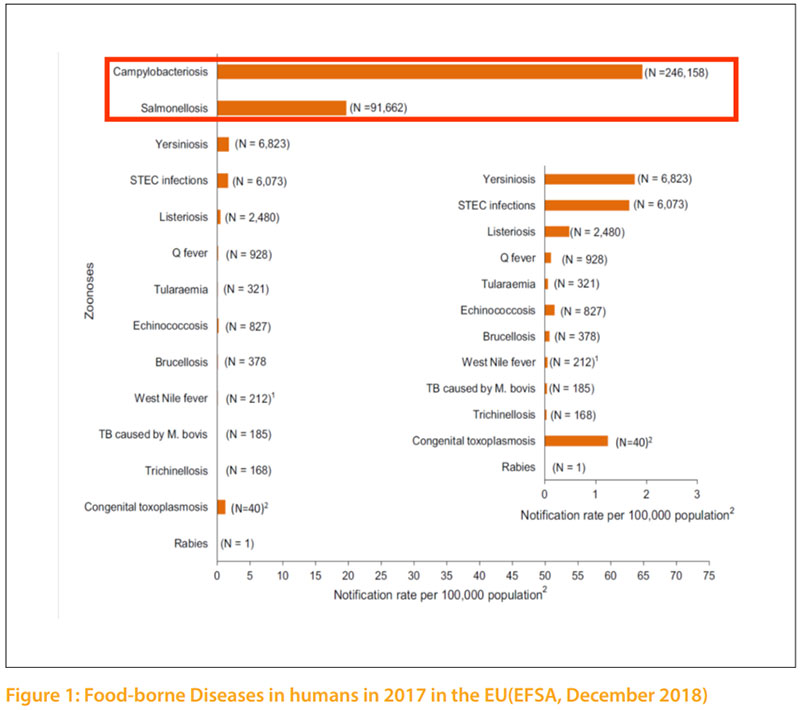
Salmonella infection
Salmonella infections in poultry are distributed worldwide and result in severe economic losses when no effort is made to control them. In poultry, the genus Salmonella of the family Enterobacteriaceae, which include more than 2500 serovars, can roughly be classified into three categories or groups as follow: Salmonella can also be divided into three groups based on their host specificity and invasiveness (Hafez, 2013). Invasive salmonellas have the capability to “invade” the body from the intestinal lumen and thus infect organs, causing more serious disease. Group 1 contains serovars, which are highly host adapted and invasive. Examples are S. Gallinarum and S. Pullorum in poultry or S. Typhi in humans. Group 2 contains non-host adapted and invasive serovars. Salmonella in this group are of most concern regarding public health, since some of them are capable to infect humans and food producing animals and especially poultry can serve as reservoirs. There are approximately 10 – 20 serovars in this group. Currently, the most relevant serovars of them are S. Typhimurium, S. Enteritidis, S. Heidelberg, S. Hadar as well as S. Arizonae. Figure 2 shows the distribution of S. Enteritidis among food and animal sources, which are closely related.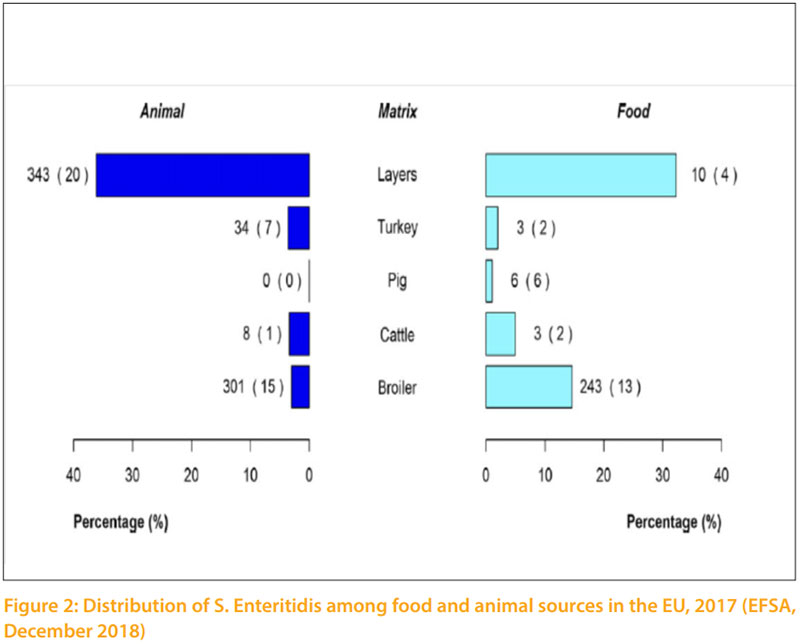 Group 3 contains non-host adapted and non-invasive serovars, which are harmless for animals and humans. Most serovars of the genus salmonella belong to this group. Some serovars may be predominant for a number of years in a region or country. Then, they disappear and are replaced by another serovars (Hafez and Hauck, 2016). The infection can be transmitted vertically through contaminated eggs laid by infected carriers as well as horizontally spread (lateral). Hatcheries are one of the major sources of early horizontal transmission. Horizontal spread of Salmonella occurring during the hatching was shown in chickens, when contaminated and Salmonella-free eggs were incubated together. Salmonella can also spread through the hatchery by means of contamination of ventilation ducting, belt slots or door seals within hatchers, but may also result from infection and contamination that continuously recycles between hatchers, hatched birds, dust and crate washing equipment. During rearing the infection is transmitted horizontally (laterally) by direct contact between infected and uninfected flocks, and by indirect contact with contaminated environments through ingestion or inhalation of Salmonella organisms. Subsequently, there are many possibilities for lateral spread of the organisms through live and dead vectors. Transmission frequently occurs via faecal contamination of feed, water, equipment, environment and dust in which Salmonella can survive for long periods. Failure to clean and disinfect properly after an infected flock has left the site can result in infection of the next batch of birds. Significant reservoirs for Salmonella are man, farm animals, pigeons, waterfowl and wild birds. Rodents, pet’s insects and litter beetles (Alphitobius diaperinus) are also potential reservoirs and transmit the infection to birds and between houses (Roche et al., 2009). Probably one of the most common sources for lateral spread
of the organisms is feed. Nearly every ingredient ever used in the manufacture of poultry feedstuffs has been shown at one time or another to contain Salmonella. The organism occurs most frequently in protein from animal products such as meat and bone meal, blood meal, poultry offal, feather meal and fishmeal. Protein of vegetable origin has also been shown to be contaminated with Salmonella (Hafez et al., 1997; Dutta et al., 2010).
Since November 2003, several regulations from the European Parliament Council Regulation on the control of salmonella and other specified food-borne zoonotic agents were passed. This regulation covers the adoption of targets for the reduction of the prevalence of specified zoonosis in animal populations at the level of primary production, including breeding flocks (Chickens and turkeys), layers, broiler and turkey flocks. Food business operators must have samples taken and tested for the zoonosis and zoonotic agents especially Salmonella (Table 1) as summarized by Hafez (2010).
Group 3 contains non-host adapted and non-invasive serovars, which are harmless for animals and humans. Most serovars of the genus salmonella belong to this group. Some serovars may be predominant for a number of years in a region or country. Then, they disappear and are replaced by another serovars (Hafez and Hauck, 2016). The infection can be transmitted vertically through contaminated eggs laid by infected carriers as well as horizontally spread (lateral). Hatcheries are one of the major sources of early horizontal transmission. Horizontal spread of Salmonella occurring during the hatching was shown in chickens, when contaminated and Salmonella-free eggs were incubated together. Salmonella can also spread through the hatchery by means of contamination of ventilation ducting, belt slots or door seals within hatchers, but may also result from infection and contamination that continuously recycles between hatchers, hatched birds, dust and crate washing equipment. During rearing the infection is transmitted horizontally (laterally) by direct contact between infected and uninfected flocks, and by indirect contact with contaminated environments through ingestion or inhalation of Salmonella organisms. Subsequently, there are many possibilities for lateral spread of the organisms through live and dead vectors. Transmission frequently occurs via faecal contamination of feed, water, equipment, environment and dust in which Salmonella can survive for long periods. Failure to clean and disinfect properly after an infected flock has left the site can result in infection of the next batch of birds. Significant reservoirs for Salmonella are man, farm animals, pigeons, waterfowl and wild birds. Rodents, pet’s insects and litter beetles (Alphitobius diaperinus) are also potential reservoirs and transmit the infection to birds and between houses (Roche et al., 2009). Probably one of the most common sources for lateral spread
of the organisms is feed. Nearly every ingredient ever used in the manufacture of poultry feedstuffs has been shown at one time or another to contain Salmonella. The organism occurs most frequently in protein from animal products such as meat and bone meal, blood meal, poultry offal, feather meal and fishmeal. Protein of vegetable origin has also been shown to be contaminated with Salmonella (Hafez et al., 1997; Dutta et al., 2010).
Since November 2003, several regulations from the European Parliament Council Regulation on the control of salmonella and other specified food-borne zoonotic agents were passed. This regulation covers the adoption of targets for the reduction of the prevalence of specified zoonosis in animal populations at the level of primary production, including breeding flocks (Chickens and turkeys), layers, broiler and turkey flocks. Food business operators must have samples taken and tested for the zoonosis and zoonotic agents especially Salmonella (Table 1) as summarized by Hafez (2010).
Campylobacters
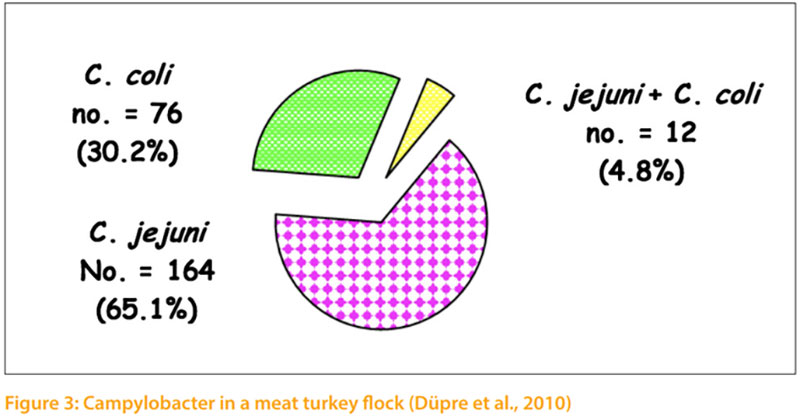
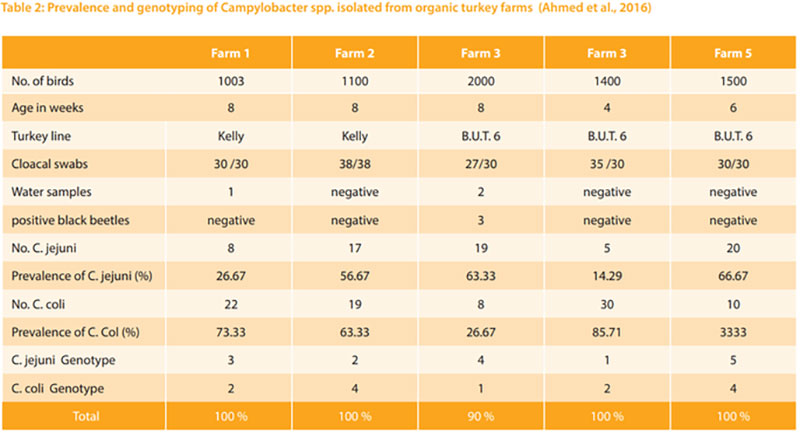
Thermophilic campylobacters are the most common bacterial cause of diarrhoea in humans worldwide. Enteric diseases caused by the thermophilic species C. jejuni, C. coli, C. lari, and C. upsaliensis range from asymptomatic infections to severe inflammatory bloody diarrhoea. The natural habitat of thermophilic Campylobacter is the intestinal tract of healthy birds and raw meat that can be contaminated during the slaughtering process (EFSA, 2015). It is estimated that as many as 90% of broilers and turkeys may harbour Campylobacter while showing little or no clinical signs of illness (Sahin et al., 2002). Hafez et al. (2001) investigate 10 turkey flocks for the presence of Campylobacter. Faecal samples were collected weekly from 1st week of age until slaughter. All monitored turkey flocks were positive for Campylobacter. Three flocks (30.0%) appeared to be infected with only one biotype. In the other 7 flocks (70.0%) two or more different biotypes were isolated during the rearing period and in slaughtered house too. In addition, Düpre et al. (2010) examined bacteriologically 1167 boot swabs taken from 161 fattening turkeys for Campylo bacter spp. Campylobacter spp. were isolated from 56 % of the investigated farms. 65.1 % of isolates were C. jejuni and 30.2% were C. coli. From 4.8% of positive samples C. jejuni as well as C. coli were isolated (Fig 3). Furthermore, Ahmed et al. (2016) examined the prevalence, genotyping and risk factors of thermophilic Campylobacter spreading in organic turkey farms in Germany. Campylobacter spp. were detected in cloacal swabs in all 5 turkey flocks with prevalence ranging from 90.0 to 100 %. 13 cloacal swabs from birds in two farms harboured mixed population of thermophilic campylobacters. In total, from 158 Campylobacter isolated from turkeys 89 (56.33 %) were identified as C. coli and 69 (43.76 %) as C. jejuni. Three Campylobacte (2 C. jejuni and 1 C. coli) were detected in drinkers of two farms and 3 C. coli were isolated from darkling beetles of one farm. No Campylobacter were isolated from main water tanks. The genotype of campylobacters isolated from wate samples or beetles were identical with those isolated from turkeys. (Table 2)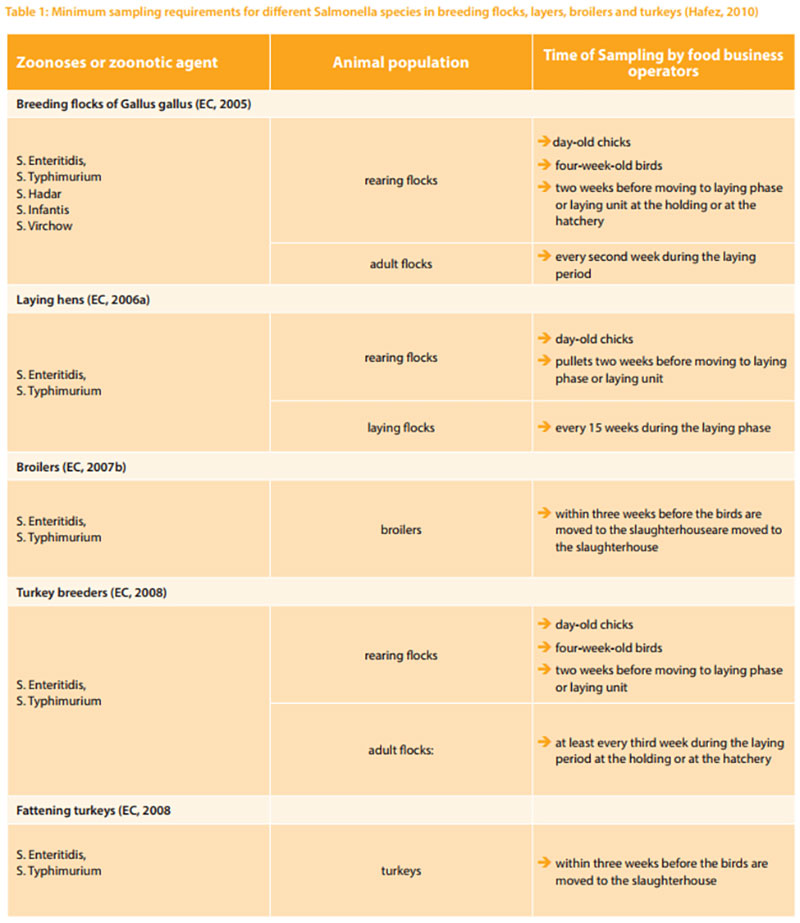
 Poultry and poultry products remain the most common source of foodborne human campylobacteriosis. The major route for Campylobacter infection in poultry appears to be the horizontal transmission from the environment. Specific flocks that become infected show rapid rate of intra-house transmission and a high isolation rate from caecal swabs, water and litter. Campylobacter spp. are widespread in poultry not only during the growing period, but also on the poultry meat during slaughter and during processing of poultry products. Horizontal transmission is the most important mode of the introduction of Campylobacter into poultry flocks. However, the ability of Campylobacter to spread is limited by their relatively low tenacity, which can vary between strains. Especially dry environments kill Campylobacter within one or two hours (Evans and Sayers, 2000).
Poultry and poultry products remain the most common source of foodborne human campylobacteriosis. The major route for Campylobacter infection in poultry appears to be the horizontal transmission from the environment. Specific flocks that become infected show rapid rate of intra-house transmission and a high isolation rate from caecal swabs, water and litter. Campylobacter spp. are widespread in poultry not only during the growing period, but also on the poultry meat during slaughter and during processing of poultry products. Horizontal transmission is the most important mode of the introduction of Campylobacter into poultry flocks. However, the ability of Campylobacter to spread is limited by their relatively low tenacity, which can vary between strains. Especially dry environments kill Campylobacter within one or two hours (Evans and Sayers, 2000).Antibiotic resistant
The development of antibiotic resistance in bacteria, which are common in both animals and humans, is an emerging public health hazard. Controlling of the above mentioned foodborne micro-organisms requires a broader understanding of how microbial pathogens enter and move through the food chain, as well as the conditions that promote or inhibit growth for each type of organism.
Multi-resistant bacteria are increasingly posing a hazard to human and animal health worldwide, impeding successful antibacterial treatment (Arias et al., 2010; EFSA, 2017). In addition, the development of novel antibiotics does not keep step with the emergence of antimicrobial resistance in bacteria (García-Rey, 2010). El- Adway et al. (2012) investigated 76 Camyplobacter jejuni isolates recovered from 67 epidemiologically unrelated meat turkey flocks in different regions of Germany in 2010 and 2011. Only one isolate was sensitive to all tested antibiotics. The numbers of isolates that were sensitive to streptomycin, erythromycin, neomycin, and amoxicillin were 69 (90.8%), 61 (80.2%), 58 (76.4%), and 44 (57.9%), respectively. The emergence of a high resistance rate and multidrug resistance to three or more classes of antimicrobial agents were observed. The resistance against sulphamethoxazole/trimethoprim, metronidazole, ciprofloxacin, naladixic acid, and tetracycline was 58 (76.3%), 58 (76.3%), 53 (69.7%), 51 (67.1%), and 42 (55.3%), respectively. Multidrug resistance to three or more classes of antimicrobial agents was found and ranged from 3.9% to 40.8%. Similar results were also found by examination of isolates collected from different free-range turkey flocks in Germany (El-Adway et al., 2015). ESBL-producing Enterobacteriaceae have emerged as pathogens in both poultry and humans (Bradford, 2001, Rawat and Nair, 2010, Moawad et al., 2018). Among multi-resistant bacteria, vancomycin-resistant enterococci (VRE) have been estimated as one of the most common bacteria causing a rise in cases of nosocomial infections in humans in the last few years (Arias et al., 2010). The prevalence of VRE in 20 turkey flocks reared in the southwest of Germany was investigated. Enterococci were tested on the presence of the vancomycin resistance genes vanA, vanB (B1/B2/B3), and vanC (C1/C2/C3). Vancomycin-resistant enterococci were detected in 15 (75%) of the 20 turkey flocks investigated. In a total of 68 isolates from birds and dust samples, enterococci bearing van-genes were detected. Of these, 12 isolates carried the vanA gene (17.6%) and 56 isolates carried the vanC1 gene (82.6%). Neither vanB (B1, B2, B3) genes nor the vanC2 or vanC3 genes could be detected (Sting et al., 2013)
Maasjost et al. (2015) investigated the antimicrobial susceptibility patterns of Enterococcus faecalis and Enterococcus faecium isolated from poultry flocks in Germany and they found that high resistance rates were identified in both Enterococcus species for lincomycin (72%–99%) and tetracycline (67%–82%). Half or more than half of Enterococcus isolates were resistant to gentamicin (54%–72%) and the macrolide antibiotics erythromycin (44%–61%) and tylosin-tartate (44%–56%). Enterococcus faecalis isolated from fattening turkeys showed the highest prevalence of antimicrobial resistance compared to other poultry production systems.
In addition, Livestock-associated methicillin-resistant Staphylococcus aureus (LAMRSA) have been isolated from a number of livestock species and persons involved in animal production. Turkey meat was also showed to be contaminated with MRSA (De Boer et al., 2009). Richter et al. (2012) investigated the prevalence of LAMRSA in fattening turkeys and people living on farms that house fattening turkeys. MRSA was detected in 18 (90%) of the 20 turkey flocks investigated. Out of examined 59 nasal swabs from persons working on the farms 22 samples were MRSpositive (37.3%). None of these persons showed clinical symptoms indicative of an MRSA infection. People with frequent access to the stables were more likely to be positive for MRSA. Similar results were about MRSA in turkeys were published by El-Adway et al. (2016).
General approaches to control food borne infections
To control the food borne organisms, information is required to understand more fully, how microbial pathogens enter and move through the food chain, and the conditions, which promote or inhibit growth for each type of organism. In general, the main strategy to control food borne infections in poultry should include monitoring, cleaning the production chain from the top, especially for vertically transmitted microorganism such as Salmonella by culling infected breeder flocks, hatching egg sanitation and limiting introduction and spread of infections at the farm level through effective hygiene measures (Hafez, 1999, 2005, Mueller-Doblies et al., 2010). An intensive and sustained rodent ontrol is essential and needs to be well planned and routinely performed and its effectiveness should be monitored. In addition, reducing bacterial colonization by using feed additives such as short chain organic acids (formic acid, propionic acid), carbohydrates (lactose, mannose, galactose, saccharose), probiotics, competitive exclusion (Schneitz, 2005, Vicente et al., 2007) or use of vaccines are further possibilities. Live and inactivated vaccines are used to control Salmonella in poultry (Gast, 2013). Generally, vaccination alone is of little value, unless it is accompanied by improvements in all aspects of management and biosecurity. In addition, further attention must be paid to the development of efficient vaccines against campylobacter infections. Since the success of any disease control programme depends on the farm and personal sanitation, it is essential to incorporate education programmes about microorganisms, modes of transmission as well as awareness of the reasons behind such control programmes by people involved in poultry production. In addition, effective education programmes must be implemented to increase public awareness of the necessary measures to be taken for protection against bacteria in food products from poultry. In spite of significant improvements in technology and hygienic practices at all stages of food production, accompanied by improvement in public sanitation, food borne infections remain a persistent threat to human and animal health. Since many humans fail to apply hygienically acceptable food handling and cooking practices, and since processing plants are not able to reduce the level of pathogenic bacteria in poultry products, every effort must be made to reduce the Salmonella contamination of live birds before despatch to processing plants. New approaches to the problem of contamination must be adopted and the discussion on the decontamination of the end product must be reevaluated carefully and without emotion. In addition, research must continue to find additional control and preventive means. As a long term contribution, poultry lines genetically resistant to some pathogens should be developed.Conclusions
-
Toward food safety in the EU several legislations are into force and their aims can be summarized according to Mulder (2011) as follows:
- Safety (consumer health): by new methods to reduce the use of antibiotics /medicines; improve disease resistance; zoonosis control; traceability of animals and products
- Safety (product safety): stimulate and control hygienic processing, traceability of products and materials intended to come into contact with food
- Animal welfare: animals kept according to rules/systems
- Product quality: improved quality and composition; quality and chain control systems; traceability of animals and products.
- Environment: reducing environmental contamination, Nitrogen and Phosphorous. There is a critical look at the use of by-products of human food production. The re-use of byproducts for non-food applications (feathers) should be encouraged.
- Rural impact, economic effects and bio-diversity

References
Ahmed M. F. E., El-Adawy, H., Hotzel, H., Tomaso, H., Neubauer, H., Kemper, N., Hartung J. and Hafez H. M. (2016). Prevalence, genotyping and risk factors of thermophilic Campylobacter spreading in organic turkey farms in Germany. Gut Pathog (2016) 8:28. DOI 10.1186/s13099-016-0108-2.Arias, C.A., Contreras, G.A. and Murray, B.E. (2010). Management of multidrug-resistant enterococcal infections. Clinical Microbiology and Infection 16:555–562.
Bradford, P.A. (2001).Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev.;14:933–951.
De Boer, E., Zwartkruis-Nahuis, J.T.M., Wit, B., Huijsdens, X.W., de Neeling, A.J., Bosch, T., van Oosterom, R.A.A., Vila, A. and Heuvelink, A.E. (2009). Prevalence of methicillinresistant Staphylococcus aureus in meat. International Journal of Food Microbiology 134: 52–56.
Düpre, S., El-Adawy, H. Hauck, R., Hotzel, H. and Hafez, H.M. (2010). Ein Beitrag zum Vorkommen von Campylobacter bei Mastputen. 80. Fachgespräch über Geflügelkrankheiten. Deutsche Veterinärmedizinische Gesellschaft (DVG): Tagung der Fachgruppe Geflügelkrankheiten ISBN 978-3-86345-046-5. Hannover. pp. 29-35..
Dutta, T.K., Roychoudhury, P. and Bandypadhyay, S. (2010). Molecular epidemiology and virulence properties of Salmonella Enteritidis isolated from an outbreak of gastroenteritis in turkeys. Indian Journal of Animal Sciences 80: 391-397.
EFSA (2018) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA Journal 2018; 16(12):5500.
EFSA (2015) The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2012. EFSA Journal 2015; 12:3547. relating to machinery. Official Journal, 1989; L 183: 9-32.
El-Adawy, H., Ahmed, M., Hotzel, H., Tomaso. H., Tenhagen, B., Hartung. J., Neubauer, H. and Hafez, H. M. (2015). Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli recovered from organic turkey farms in Germany. Poultry Science, 11:2831-2837.
El-Adawy, H., Ahmed, M., Hotzel, H., Monecke, S. Schulz, J., Hartung, J., Ehricht, R., Neubauer H. and Hafez, H.M. (2016). Characterization of Methicillin-Resistant Staphylococcus aureus isolated from healthy turkeys and broilers using DNA Microarrays. Frontier in Microbiology, 19 December 2016 | https://doi.org/10.3389/ fmicb.2016.02019.
El-Adawy, H., Hotzel, H., Düpre, S., Tomaso, H., Neubauer, H. and Hafez, H. M. (2012). Determination of antimicrobial sensitivities of Campylobacter jejuni isolated from commercial turkey farms in Germany. Avian Diseases, 56:685–692.
Evans, S. and Sayers, A. (2000). A longitudinal study of campylobacter infection of broiler flocks in Great Britain. Preventive Veterinary Medicine, 46(3):209-223.
García-Rey, C. (2010). The role of the pharmaceutical industry. Why are new antibiotics not being marketed? . Enfermedades Infecciosas Y Microbiologia Clinica, 28:45-49.
Gast, R.K. (2013). Paratyphoif infections. In Diseases of Poultry, D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez and V. Nair, eds. (Ames, Iowa: Iowa State Press), pp. 693–706.
Hafez, H.M. (1999). Poultry meat and food safety: pre- and post-harvest approaches to reduce food borne pathogens. World’s Poultry Science Journal, 5: 269-280.
Hafez, H.M. (2013). Salmonella infections in Turkeys. In: PA Barrow, P.A. and Methner, U. (Ed). Salmonella in domestic animals. CABI Publishing ISBN 13: 978 1 84593 902 1. pp.193-220.
Hafez, H.M. (2005). Governmental regulations and concept behind eradication and control of some important poultry diseases. World’s Poultry Science Journal, 61:569-582.Hafez, H.M. (2010). Salmonella Infections in Poultry: Control approaches in the European Union. Turkiye Klinikleri J Vet Sci; 1(2):80-88. https:// doi:10.2903/j.efsa.2017.4694.
Hafez, H.M. and Hauck, R. (2016). Salmonellosis. In: Hafez, H.M. and Hauck R (Ed). Main Diseases in poultry farming – Bacterial infection. Publisher Grupo Asís Biomedia, S.L.- Spain. ISBN: 978-84-16818-39-6. pp. 11-23.
Hafez, H.M., Schroth, S., Stadler, A. and Schulze, D. (2001). Detection of salmonella, campylobacter and verotoxin producing E. coli in turkey flocks during rearing and processing. Archiv für Geflügelkunde 65,130 –136.
Hafez, H.M., Stadler, A. and Kösters, J. (1997). Surveillance on Salmonella in Turkey Flocks and Processing Plants. Deutsche Tierärztliche Wochenschrift, 104, 33-35.
Moawad, A.A., H. Hotzel, H. Neubauer, R. Ehricht, S. Monecke, H. Tomaso, H. M. Hafez, U. Roesler and H.El Adawy (2018). Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: emergence of colistin -resistant and extended -spectrum β –lactamase -producing Escherichia coli. Gut Pathog (2018) 10:39. https://doi. org/10.1186/s13099-018-0266-5
Maasjost, J., Mühldorfer, K. Cortez de Jäckel S. and. Hafez, H. M. (2015) Antimicrobial Susceptibility Patterns of Enterococcus faecalis and Enterococcus faecium isolated from poultry flocks in Germany. Avian Diseases, 59:143–148.
Mueller-Doblies, D., Carrique-Mas, J.J., Sayers, A.R. and Davies, R.H. (2010). A comparison of the efficacy of different disinfection methods in eliminating Salmonella contamination from turkey houses. Journal of Applied Microbiology, 109: 471-479.
Mulder, R. (2011). Current EU regulations for the production and processing of (safe) poultry meat. http://en.engormix.com/MA-poultry-industry/health/ articles/current-regulations-production-processingt1774/165-p0.htm.
Richter, A., Sting, R., Popp, C., Rau, J., Tenhagen, B.A., Guerra, B., Hafez, H.M. and Fetsch, A. (2012) Prevalence of types of methicillin-resistant Staphylococcus aureus in turkey flocks and personnel attending the animals. Epidemiology and Infection, 140, 2223- 2232.
Rawat, D. and Nair, D. (2010). Extendedspectrum beta-lactamases in Gram negative bacteria. J Glob Infect Dis. 2010; 2: 263–74.
Roche, A.J., Cox, N.A., Richardson, L.J., Buhr, R.J., Cason, J.A., Fairchild, B.D., and Hinkle, N.C. (2009). Transmission of Salmonella to broilers by contaminated larval and adult lesser mealworms, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Poultry Science, 88: 44-48.
Sahin, O., Morishita, T., Zhang, and Q. (2002). Campylobacter colonization in poultry: sources of infection and modes of transmission. Animal Health Research Reviews, 3(02):95-105.
Schneitz, C.: (2005) Competitive exclusion in poultry-30 years of research. Food Control,2005; 16: 657-667.
Sting, R., Richter, A., Popp, C. and Hafez, H.M. (2013). Occurrence of vancomycinresistant enterococci in turkey flocks. Poultry Science, 92:346–351.
Vicente, J., Higgins, S., Bielke, L., Tellez, G., Donoghue, D., Donoghue, A., and Hargis, B. (2007). Effect of probiotic culture candidates on salmonella prevalence in commercial turkey houses. Journal of Applied Poultry Research, 16:471-476.










