Abstract
Between 8th November 2016 and 31th May 2017, more than 1,150 cases of HPAI H5N8 in wild birds and 107 out-breaks in birds kept in captivity (92 poultry holdings and 15 zoos/animal parks) were reported in Germany. This HPAI epidemic is the most severe recorded in Germany so far. In total, 29 European states have been affected. In addition to H5N8, two other HPAI subtypes have been detected in Europe, i.e. HPAI H5N5 (in seven states) and H5N6 (in Greece). The viruses were probably introduced by migratory birds, sparking an epidemic among wild birds with occasional incursions into poultry holdings, zoos and animal parks, which were usually rapidly controlled. In many cases, substantial gaps in farm biosecurity may have eased the path for virus entry. Only in the late phase of the epidemic, there was also epidemiological and molecular evidence for transmission of the infections between commercial holdings, which caused approximately 20-25 percent of the total number of outbreaks. Biosecurity measures should be checked and maintained at a high level. This includes, inter alia, wearing of stable specific protective clothing and footwear, cleaning and disinfection of equipment that has been in contact with birds and the best possible prevention of contacts between poultry and wild birds.
Keywords
Avian influenza, H5N8, fowl plague, epidemiology, Germany, biosecurityCo-Authors
Globig A, Sauter-Louis C, Dietze K, Homeier T, Probst C, Gethmann J, Depner K, Wysocki P, Reimer N, Staubach C, Grund C, Harder T, Starick E, Pohlmann A, Höper D, Beer M, Mettenleiter TC, Conraths FJ
Backgroun
Avian Influenza, colloquially called ‘bird flu’, is an infectious disease in poultry caused by influenza A viruses, which are enveloped viruses with a segmented singlestranded RNA genome. They belong to the family Orthomyxoviridae. These viruses occur in two variants (low/highly pathogenic) and different subtypes. Wild water birds (Anseriformes) as well as gulls, terns and wader birds (Charadriiformes) are regarded as the natural reservoir for all low pathogenic avian influenza viruses (LPAIV), i.e. viruses of the subtypes H1-H16 and N1- N9. LPAIV of the subtypes H5 and H7 may cause almost no or only mild disease in domestic poultry. Particularly in ducks and geese, the infection may be asymptomatic or lead to very mild symptoms. However, these subtypes have the capacity to evolve spontaneously into a highly pathogenic form (highly pathogenic avian influenza viruses, HPAIV), which usually happens in domestic poultry after transmission of the low pathogenic progenitors from wild birds (Jones et al., 2012). The highly pathogenic form clinically manifests in poultry as fowl plague, which causes drastic losses especially in turkeys and chickens. In ducks and geese, however, the clinical signs of an HPAIV infection may be mild and mortality can be lower than in turkey and chickens. Therefore, HPAI viruses may circulate in waterfowl undetected, whereas mortality is always very high in Galliformes (75- 100%; Swayne and Suarez, 2000).Upon exposure to a high infectious dose, usually after direct contact to infected birds, some avian influenza viruses (e.g. HPAIV H5N1 and H5N6, LPAIV H7N9 in China, of which a HPAI variant has recently been detected), can be transmitted to humans and have the potential to cause fatal human disease. Due to the segmented genome of influenza A viruses, new viruses can evolve when simultaneous infections of a single host with different influenza A viruses allow mixing (reassortment) of the genome segments. Therefore, there is a permanent risk for the generation of novel influenza viruses with pandemic potential if different influenza A virus strains cocirculate (Lam et al., 2010).
In 1996, an HPAIV of subtype H5N1 originating from geese (goose/Guangdong/96) caused outbreaks in chickens and disease in 18 humans with six fatalities. This virus subsequently evolved steadily and extremely successfully during the following two decades into various phylogenetic clades, subtypes and numerous genotypes. A combination of vaccination of poultry against HPAI H5, live bird markets and the traditional way of keeping waterfowl, for example in rice fields, in contact to wild birds is a perfect source for the genesis, emergence and evolution of new HPAIV in large parts of Asia, especially in South East Asia. Wild birds mixing with poultry may contribute to the development of new viruses by reassortment and to the intra- and intercontinental spread of newly reassorted viruses. Many of the H5- descendants caused serious outbreaks of fowl plague in poultry in South East Asia and some managed to find their way to Europe: in 2005/2006 (H5N1 clade 2.2), in 2010 (H5N1 clade 2.3.2.1c) and 2014 (H5N8 clade 2.3.4.4a). This development has led to a massive increase of HPAI outbreaks worldwide since 1996 (Guan and Smith 2013; Lee et al., 2016). Some, but not all, of these HPAI H5 strains can cause clinical infections also in humans. The generation of a potentially pandemic virus that is able to spread among humans is of worldwide concern and under careful observation.
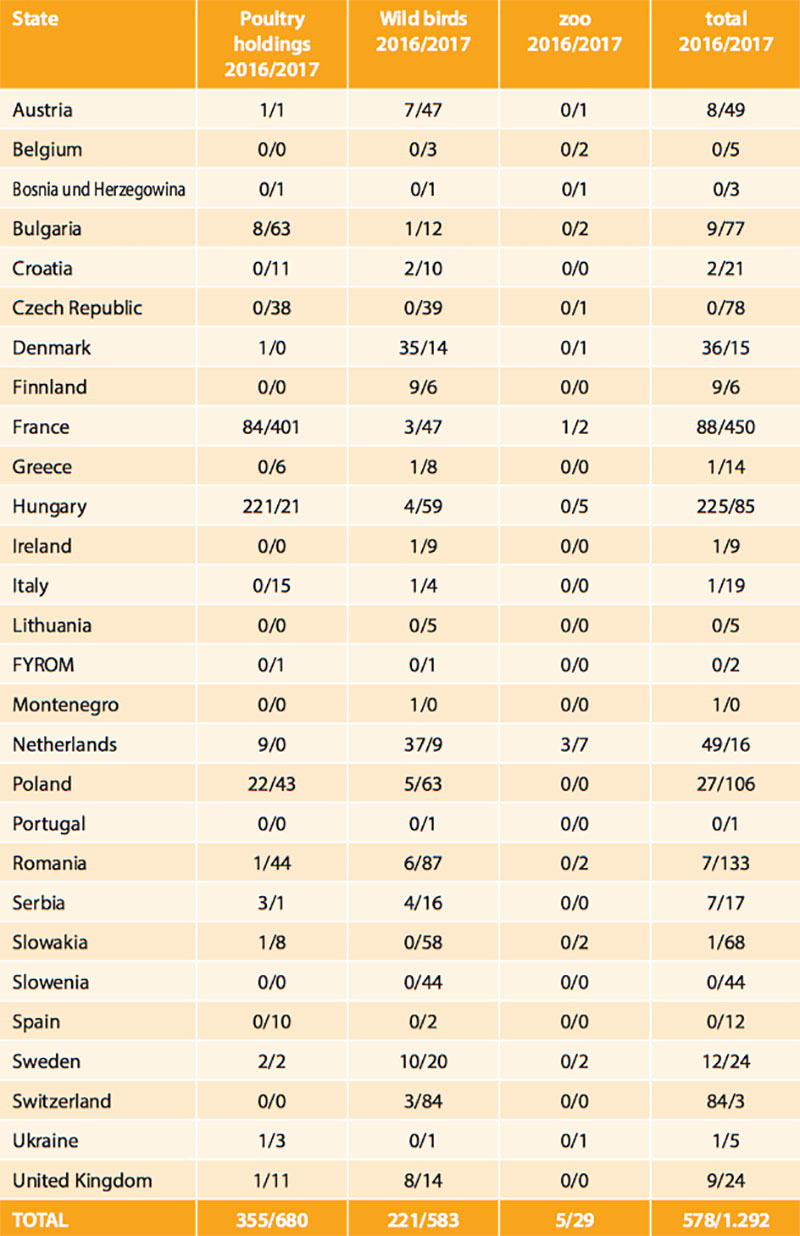
Table 1: HPAI outbreaks in poultry and number of locations of HPAI infections in wild birds in European states in 2016 and 2017
HPAI H5N8 CLADE 2.3.4.4 B outbreaks in Europe an Germany
In September 2016, the FAO released a risk alert about the potential westward spread of a novel HPAI H5N8 virus of clade 2.3.4.4 B, which was detected through surveillance of wild migratory birds in the Tyva Republic, Russian Federation (Sims et al., 2016). Only one month later Hungary and then also Poland notified the first cases of HPAI H5N8 clade 2.3.4.4 b in dead wild birds (a swan in Hungary and ducks as well as gulls in Poland).A few days later, on 7 November 2016, an increased mortality of uncertain cause was first reported in tufted ducks (Aythya fuligula) at Lake Constance. One day later, on 8 November, HPAI H5N8 was confirmed in dead wild birds (mostly tufted ducks) at Lake Constance in Baden-Wuerttemberg, Southern Germany, as well as in tufted ducks found dead at Lake Plön in Schleswig- Holstein, Northern Germany. Simultaneously, an increased number of water birds and sea gulls were found dead at the Eastern coast of Schleswig-Holstein, around Lake Constance in Switzerland, Austria and Germany (Bavaria and Baden- Wuerttemberg) as well as in Mecklenburg- Western Pomerania, North-Eastern Germany. Genetic analysis and animal experiments showed later that there was no indication of a zoonotic potential of the H5N8 virus (EFSA, 2017) and no human infections with this virus have indeed been reported so far.
Soon, the epidemic nature of the HPAI H5N8 strain for birds became obvious as it spread across Germany affecting mainly wild waterbirds overwintering at lakes and rivers or along the coast, and scavenging birds (white-tailed sea eagles, buzzards, crows, gulls, owls, etc.) that had apparently fed on infected carcasses. The virus was isolated from at least 53 wild bird species. Besides Germany, 28 other European states have been hit by the epidemic (Table 1, Figure 1). A severe outbreak series occurred particularly in France, but also in Bulgaria, Romania, Poland, Czech Republic and Hungary, where many domestic duck and geese flocks were infected. To control the circulation of various HPAI and LPAI H5 viruses including HPAIV H5N8 in domestic waterfowl in South-Western France, the government took drastic measures including the depopulation of all kept waterfowl and prohibition of keeping ducks and geese for some time in regions, where infections were mainly detected by surveillance.
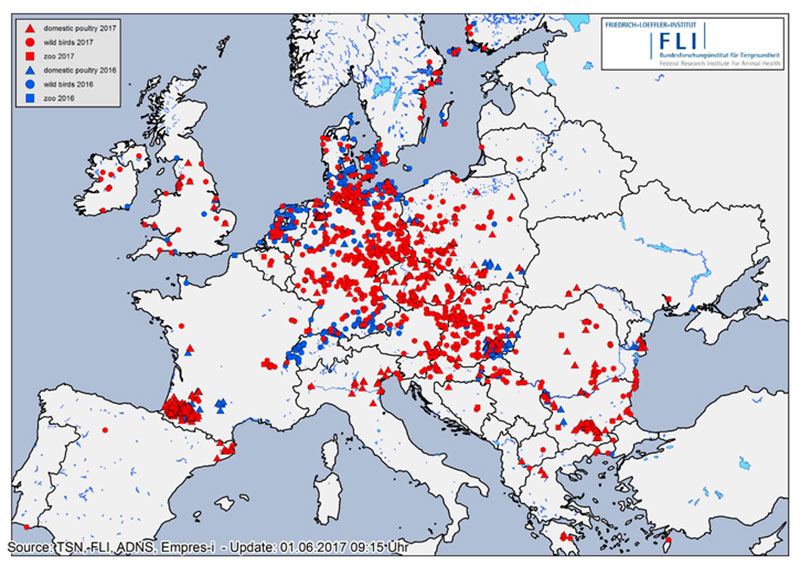
Figure 1: Confirmed cases of HPAI in Europe in 2016 (blue symbols) and 2017 (red symbols) in wild birds (circles), poultry (triangles) and in animal parks/zoological gardens (squares) registered in ADNS (Animal Disease Notification System of the European Union), Empres-i (FAO) or TSN (German Animal Disease Notification System).
Between 8th November 2016 and 15th May 2017, more than 1,150 cases of HPAI H5N8 in wild birds and 107 outbreaks in birds kept in captivity (92 poultry holdings and 15 zoos or animal parks) were reported in Germany. The last outbreak in poultry was reported on 9th May 2017. Thus, the HPAI epidemic seemed to be waning in Germany since April 2017 most likely due to a rise in temperature and increasing UV radiation as well as due to lower densities of overwintering waterfowl on lakes and rivers. This is the most severe epidemic of HPAI recorded in Germany so far. Generally, the temporal course of the epidemic in wild birds was characterized by at least two waves, with maxima in mid- November 2016 and mid-February 2017, respectively (Figure 2). A few days after the detection of HPAIV H5N8 in wild birds, the first outbreaks were reported in noncommercial poultry (backyard) and subsequently also in large commercial poultry farms. By the end of February 2017, all federal states of Germany had reported HPAIV H5N8 infections in wild birds or poultry (Figures 1, 3). During the second wave of the epidemic, further HPAI H5-reassortants were found in wild birds and domestic poultry (turkey) in Schleswig-Holstein. These strains could be clearly distinguished from the first reported strains as they belonged to different genotypes involving several gene segments including another NA subtype (N5). Phylogenetic analyses indicated that multiple independent entries of viruses into Germany had occurred more or less at the same time (Pohlmann et al., 2017).
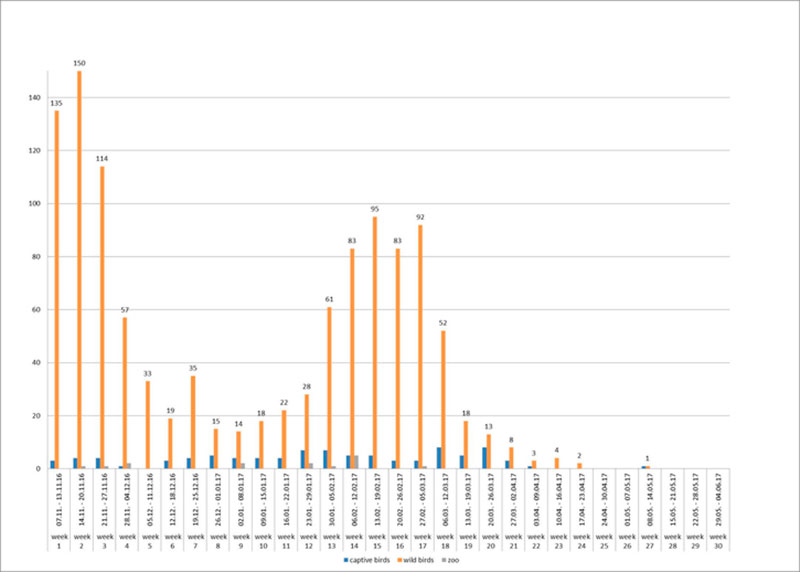
Figure 2: Weekly numbers of outbreaks of HPAI in poultry (red columns), zoos (blue columns) and cases in wild birds (green columns) in Germany (November 2016- May 2017).
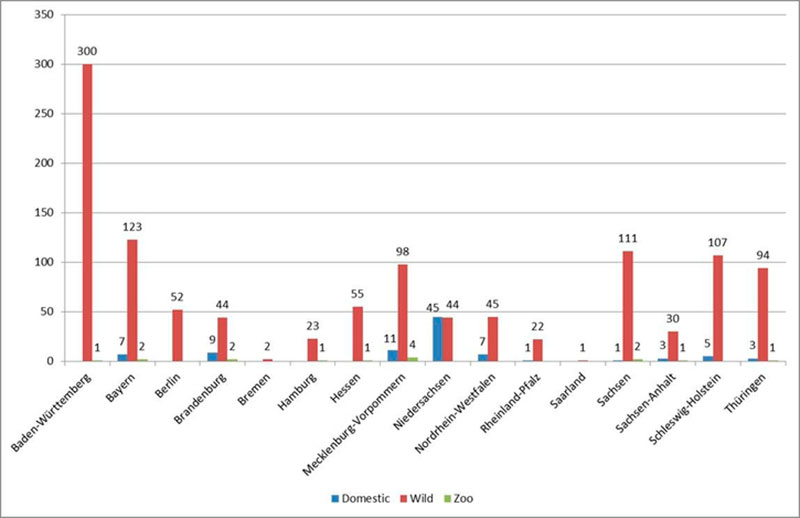
Figure 3: Numbers of reported cases in wild birds and outbreaks in poultry in the German Federal States.
Epidemiological investigations of outbreaks in poultry holdings
A total of 68 commercial poultry holdings were affected by the epidemic; thereof 52 turkey, 5 laying hen, 9 duck and 2 geese holdings (Figure 4). Moreover, 24 small scale, non-commercial poultry holdings were affected by HPAIV H5N8. They were distributed almost all over Germany and were, like the outbreaks in captive birds in zoos, most likely caused by primary virus introduction into the holdings/zoos via direct contact to infected wild birds (where captive birds were kept outdoors and with access to ponds also visited by wild birds) or via indirect contact (feces or carcass contaminated material). No links regarding trade of live animals, feed or products of animal origin were detected between the outbreaks in Germany. The majority of outbreaks in large commercial poultry holdings were apparently caused by single incursion events, often affecting only one stable. In many cases, substantial gaps in farm biosecurity may have eased the path for virus entry. Only in the late phase of the epidemic, there was also epidemiological and molecular evidence for transmission of the infections between commercial holdings, which caused approximately 20-25 percent of the total number of outbreaks.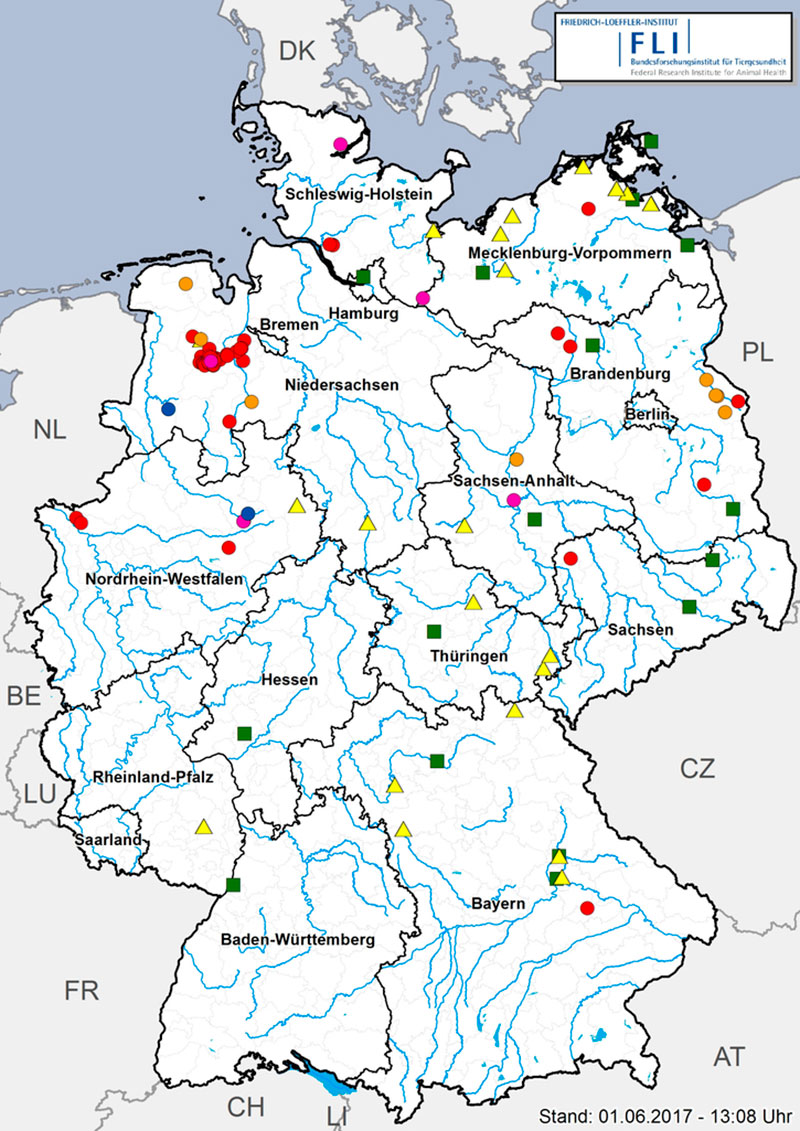
Figure 4: HPAI in captive bird holdings in Germany (08. November – 18 May 2017). Red circles: turkeys (52), orange circles: ducks (9), blue circles: geese (2), pink circles: laying hens (5), yellow triangles: small scale, mixed holdings (24), green squares: zoos (15).
Conclusions and Outlook
The simultaneous endemic circulation of various HPAI and LPAI viruses in parts of Asia and Africa in wild birds and a substantial number of outbreaks in poultry do not give an optimistic view on the chances to eradicate these viruses. On the contrary, the poultry industry, politics and poultry associations must prepare for further outbreaks in future. Fortunately, the current HPAIV H5N8 clade 2.3.4.4b had no zoonotic potential, but this can change as new viruses have evolved in Asia, which can lead to fatal infections in mammals. Vaccination has mostly complicated the situation. The only efficient measures to prevent the spread of avian influenza viruses are prompt detection of infection, closure of the holding, immediate depopulation and cleansing/disinfection of the affected holding, as well as a temporary ban on restocking (EFSA, 2017). Moreover, potential contact to wild birds, mode and frequency of farm visits, biosecurity practices, and the density of poultry holdings in a specific region are relevant risk factors for the introduction and the spread of HPAI (Ssematimba et al., 2013). Strictly-enforced farm biosecurity rules are essential to prevent the introduction and spread in poultry farms in high density areas, particularly in times of epidemics. Particularly in high risk periods and locations, losses should be fully compensated only if biosecurity rules had been established and enforced.Currently, the risk of introduction into domestic poultry holdings and captive bird holdings in zoological gardens through direct and indirect contacts between wild birds and domestic poultry is estimated to be moderate for Germany and Europe (FLI, 2017; DEFRA, 2017). By the end of summer, this risk will probably rise again, particularly for holdings in the vicinity of resting and gathering areas of wild waterfowl, including agricultural crop land.
Protection of domestic poultry holdings from infection with HPAIV H5N8 has the highest priority. Emphasis is put on the creation of a physical and functional barrier between wild bird habitats and domestic poultry holdings. Among other biosafety measures, mandatory indoor housing of poultry or the use of protected shelters (fenced and covered with fabric) minimize the risk of direct and indirect contact with infected wild birds. In particular, indirect introduction routes, e.g. through feed contaminated by wild birds, contaminated water, litter and objects (shoes, wheelbarrows, vehicles etc.) must be cut off and adequate disinfection measures must be taken. Revision, optimization and strict implementation of biosafety measures are of utmost importance. Poultry farmers are obliged by law to observe basic biosafety rules. The FLI has published a biosafety checklist together with the German Poultry Association (Zentralverband der Deutschen Geflügelwirtschaft e.V.), which is based on an EFSA opinion (EFSA, 2017). The document can be downloaded from https:// www.openagrar.de/servlets/MCRFileNodeServlet/ openagrar_derivate_00002067/ Checkliste-Gefluegelpest-2017-03-17.pdf (only in German). Some interesting farm biosafety guidelines and toolkits can be found under http://www.farmbiosecurity. com.au/industry/chickens/ .
Further information on the HPAIV H5 distribution (maps) and current risk assessments on the introduction of HPAI viruses to Germany is available from https://www.fli.de/ en/news/animal-disease-situation/avianinfluenza- ai-fowl-plague/ for Germany. FAO releases on a regular basis information about the spread of relevant avian influenza viruses, especially HPAI H5N1 and LPAI/ HPAI H7N9 (http://www.fao.org/avianflu/ En/index.html, http://www.fao.org/ag/ againfo/programmes/en/empres/H7N9/).

References
Guan Y, Smith GJD (2013): The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res. 178, 35-43.Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, McKeever D, Mutua F, Young J, McDermott J, Pfeiffer DU (2012): Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci 21;110(21):8399-404. doi: 10.1073/ pnas.1208059110. Epub 2013 May 13.
Department for Environment, Food and Rural Affairs animal and Plant Health Agency DEFRA (2017): Updated outbreak assessment number 13. Highly pathogenic avian influenza H5N8 in the UK and Europe. https://www.gov.uk/government/uploads/system/uploads/attachment_ data/file/607559/uoa-avian-flu-europeupdate13.pdf
EFSA AHAW Panel (2017):, Urgent request on avian influenza. EFSA Journal 15(1):4687. https://www.efsa.europa.eu/sites/default/files/4687.pdf
FLI (2017): Risk Assessment for the introduction of highly pathogenic avian influenza H5 viruses into poultry in Germany of the 17 of May 2017. https://www.fli.de/en/news/animal-disease-situation/avianinfluenza-ai-fowl-plague/
Lam, TT, Hon C, Tang JW (2010): Use of phylogenetics in the molecular epidemiology and evolutionary studies of viral infections. Critical Reviews in Clinical Laboratory Sciences, 47:1, 5-49, DOI: 10.3109/10408361003633318
Lee DH, Sharshov K, Swayne DE, Kurskaya O, Sobolev I, Kabilov M, Alekseev A, Irza V, Shestopalov A (2017): Novel Reassortant Clade 2.3.4.4 Avian Influenza A(H5N8) Virus in Wild Aquatic Birds, Russia, 2016. Emerg Infect Dis. 23 (2), 358-360.
Pohlmann A, Starick E, Harder T, Grund C, Höper D, Globig A, Staubach C, Dietze K, Strebelow G, Ulrich RG, Schinköthe J, Teifke JP, Conraths FJ, Mettenleiter TC, Beer M. (2017): Outbreaks among Wild Birds and Domestic Poultry Caused by Reassorted Influenza A (H5N8) Clade 2.3.4.4 Viruses, Germany, 2016. Emerg Infect Dis. 23 (4):633-636. doi: 10.3201/eid2304.161949. Epub 2017 Apr 15.
Sims L, Khomenko S, Kamata A, Belot G, Bastard J, Palamara E, Bruni M, von Dobschuetz S, Dauphin G, Raizman E, Lubroth J (2016): H5N8 highly pathogenic avian influenza (HPAI) of clade 2.3.4.4 detected through surveillance of wild migratory birds in the Tyva Republic, the Russian Federation – potential for international spread. Empres watch (35), September 2016. http://www.fao.org/3/a-i6113e.pdf
Ssematimba A, Hagenaars TJ, de Wit JJ, Ruiterkamp F, Fabri TH, Stegeman JA, de Jong MCM (2013). Avian influenza transmission risks: Analsysis of biosecurity measures and contact structure in Dutch poultry farming. Prev Vet Med 109 (1-2), 106-115.
Swayne DE, Suarez DL (2000): Highly pathogenic avian influenza. Rev. Sci.Tech. 19, 463-468.