Summary
Hatchability is of considerable economic importance for all hatcheries and therefore must be given
appropriate attention in breeding programs for commercial layers. The variability between and within
strains will tell us whether reproductive performance can be improved by selection within specific
lines. The aim of this study was to analyse the genetic variation of hatchability and correlations with
production traits. Data collected from two pure-bred female lines (LSL) at 45-46 weeks of age were
used. Estimated heritabilities were low (h² = 0.13 and 0.15) for fertility, but moderately high (h² = 0.27
and 0.30) for hatch of ‘fertile’ eggs (eggs transferred at 18 days). Hatchability was negatively correlated
with egg weight (rg = -0.43 to -0.52) and albumen height (rg = -0.25 to -0.42). Favourable correlations
were found with egg production (rg = -0.01 to +0.28), percentage yolk (rg= +0.08 to +0.39) and some
shell quality traits like shape index and breaking strength (rg = +0.14 to +0.32), but not with dynamic
stiffness (rg = -0.08 to -0.17).
Hatch of fertile eggs has sufficient genetic variation in these White Leghorn lines to expect improvement by within-line selection, especially if supported by a reduction in total egg weight and selection
for higher egg production, yolk percentage and shell breaking strength.

Introduction
The avian egg is a biological system intended to ensure the well-being of the embryo and its successful hatching into a fully developed chick (Narushin and Romanov, 2002). Predictable reproductive performance of parent stock is essential for every commercial hatchery to produce as many saleable chicks at the lowest possible cost, and uniform chicks set the basis for a successful rearing and subsequent production. If the conditions in the setters and hatchers are not optimized, the uniformity of chicks will suffer and the hatching time will spread out. Many factors can affect hatchability, especially egg size and age of breeders, season of the year and nutrition, egg handling and storage, temperature and humidity throughout the incubation and hatching period (Wilson 1997).Reproductive traits usually reach a peak close to peak production and decline with increasing age toward the end of the laying cycle. The decrease of hatchability in older flocks is well-known and may be explained as a result of lower fertility of males and females, but probably more important is the reduction in eggshell quality with increasing egg weight. Bamelis (2003) suggested that low hatchability of fertile eggs at the beginning of the breeding season and the decline in hatchability with increasing age is also due to improper egg water loss, which should be avoided by adjusting incubation conditions in accordance with water vapour eggshell conductance. In management guides for commercial parents, 52 to 68 g may be specified as optimal weight range for hatching eggs. The critical question is how many small eggs from young flocks and how many large eggs from old flocks are discarded to guarantee the best possible chick quality and satisfied customers.
Breeds and lines of the same breed differ in reproductive traits, but relatively little within-line selection has been practiced for hatchability in commercial breeding programs focused on efficient egg production of the final cross (Flock, 1995). Reproductive traits usually benefit from heterosis in cross-line parents. Natural selection should always favour families with above average reproductive rates, because they contribute with more progeny to the next generation. Hatchability is a typical fitness trait with low heritability, which suggests that optimization of breeder farm and hatchery management is the most promising route for improvement (Förster et al., 1992). However, low heritability does not exclude improvement by selection; it only takes a long time to see measurable results. Estimates of heritability for hatch of fertile eggs in the literature ranges from 0.02 to 0.24 (i.e. Förster, 1993; Beaumont et al., 1997; Szwaczkowski et al., 2000; Sapp et al., 2004; Bennewitz et al., 2007; Rozempolska-Rucinska et al., 2009; Sharifi et al., 2010; Wolc et al., 2010).
There is a positive correlation between egg weight and incubation time, but the incubation time is affected much more by pre-incubation storage time than by initial egg weight (Wilson, 1991). As a rule of thumb, the lengthening of the incubation time with increasing storage time is estimated to be 0.5 to 1 h of incubation for each additional storage day (Förster, 1994). Moreover, the moisture loss of the eggs during storage should be kept at a minimum; this can be reached with a high relative humidity in the air, 75-85%. Furthermore a positive effect in the hatch results after long storage is reported when the eggs are stored upside down, with the pointed end up (Mayes and Takeballi, 1984; Förster, 1994). Regularly turning of eggs during incubation is important to ensure the nutrition and fluid balance of the embryo and improves hatchability. In commercial hatcheries, eggs are turned in a 45° angle each hour thorough the setter period, although it seems to be no need for further turning after twelve days of incubation. Wilson (1991) concluded that maximum hatchability was achieved with a turning frequency of 96 times per day, but 24 times per day was a practical frequency.
Hatching time from the first to last chick on a tray often spreads over a period of 24-48 hours, and all chicks are kept in the hatcher until most chicks have hatched. Chick processing in the hatchery and transport to the destination farm may involve up to 72 hours without water and feed for some chicks, which will have negative effects on survival and later performance. The spread in individual hatch causes variability in biological age and chick quality (Tona et al., 2003; Willemsen et al., 2008). As Decuypere and Bruggeman (2007) pointed out, the spread of hatch varies due to pre-incubation factors (e.g. age of parent flock and duration and temperature during egg storage, egg turning, the gaseous environment), only some of which can be controlled with optimal management. If hatching eggs from different parent flocks are used to supply large orders, these effects should be known and taken into account.
Hatchability declines with storage duration of hatching eggs over an extended period even under optimum storage conditions (Mayes and Takeballi, 1984). Although commercial hatcheries avoid prolonged storage of hatching eggs, sometimes there is no other option to fill large orders. Tona et al. (2003) concluded that long egg storage time increases incubation duration, which affects negatively the quality of the chicks. One of the methods to reduce the negative effect of long-term storage has been to pre-incubate eggs for short periods before storage (Meijerhof, 1992).
Different lines may also respond differently to longer storage periods, i.e. some lines maintain acceptable hatchability longer than others (Förster, 1994). A good management practise to optimize hatchability is to pre-heat the eggs before they are introduced in the setters, thus reducing the temperature difference between storage and setters. With comparable humidity in the setter, large eggs lose relatively less water than small eggs, which should be taken into account by lowering humidity in the setter so that the desired weight loss is achieved.
Egg characteristics greatly influence the process of incubation and are responsible for its success (Narushin and Romanov, 2002). The egg shell has an important role during embryonic development, isolating the embryo from the external environment while allowing the proper gas exchange through the shell. Barnett et al. (2004) reported that eggs with hair-cracks showed increased bacterial exposure and weight loss, with significantly lower hatchability (56.4% vs. 80.9%) compared with intact shells. Bennet (1992) compared thin and thick shells based on specific gravity measurements and reported a reduction in hatchability of 3 to 9%, which he attributed to increased cracks, moisture loss and bacterial contamination of eggs with thin shells.
To support hatchery management in producing high quality chicks within a reduced hatch window, even from older flocks and/or prolonged egg storage, primary breeders select families with persistent hatchability under these conditions (Förster, 1994). The aim of the present study is to estimate genetic parameters for reproductive traits and to evaluate genetic relationships with egg quality and production traits so that genetic improvement of total performance including hatchability can be optimized.
Material and methods
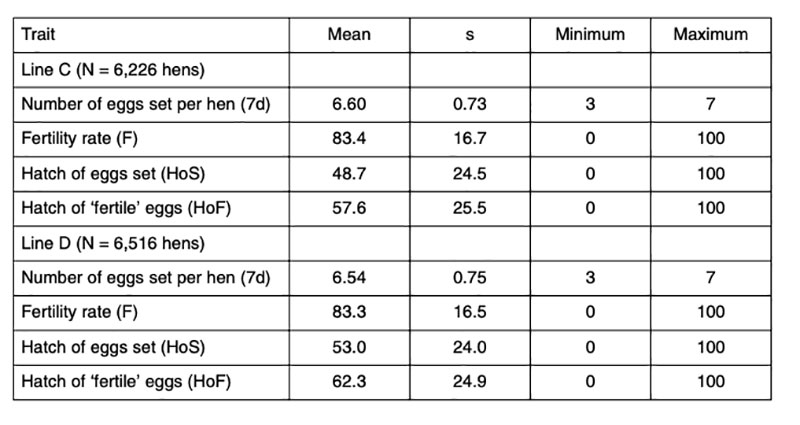
Data of 6,226 and 6,516 fully pedigreed hens of two pure-bred female lines C and D of a commercial White Leghorn breeding program (LSL) were analyzed. The average number of daughters per sire and per dam was 48.3 and 6.4 and 50.5, and 6.6 for the lines C and D respectively. Each hen was tested twice at the age of 45 and 46 weeks. Hatching eggs were collected for a period of 7 days each time. Double-yolk eggs, misshaped eggs, dirty eggs, eggs without shell or with abnormal shell were excluded. Prior to setting, the hatching eggs were subjected to long storage challenging conditions, which explains the low average hatchability (Table 1). Prolonged storage was expected to increase the apparent variation between families, thus improving the basis for selection. All eggs were stored in the hatchery for 7 days after the last collection day at 16°C and 60-70% HR, i.e. the eggs were between 8 and 15 days old when set. Furthermore, the time of incubation was limited to exactly 508 hours (21 days + 4 h), 8 hours less than commercial hatcheries would plan for these lines. Pooled semen was used from several males to eliminate the influence of male fertility. Trays for 150 eggs were used, allowing 2 hens per row (max. 7 eggs per hen and a gap in the middle), i.e. eggs of 20 hens per tray. The trays were randomly distributed in the incubators, and possible environmental differences within the machines were treated as part of the error variance.Table 1: Phenotypic statistics for the reproductive traits analysed (data from two years)
The eggs were candled on the 18th day of incubation and transferred to the hatchers after elimination of ‘clear’ eggs (infertile eggs and early embryonic mortality). True fertility was probably around 95%, based on data reported by Sharifi et al. (2010), who found fertility ranging between 94% and 97% in a similar test of data from these lines. Families with less than 3 fertile eggs were completely removed. At hatch, only first-quality chicks were taken into account, which means, that chicks which had physical abnormalities, unhealed navels or were too wet or too weak were not counted and not considered to calculate hatchability.
Data of two generations were used for this study. In each generation, three different houses were tested with two hatches. The traits recorded in this test are: fertility rate (F), hatch of eggs set (HoS) and hatch of fertile eggs (HoF). Information about egg production and egg quality of these pedigree birds was available as well. Egg quality was measured on other eggs of the same hens before and after the hatching test.
Statistical Analysis
In breeding practice there are often situations in which individual performance can be measured repeatedly, and the average of individual records can be used as selection criterion. The collection of repeated measurements and subsequent use of average performance as selection criterion can be especially advantageous in traits with low heritability but good repeatability. For the estimation of the heritability of hatchability and fertility, the mean value of the two hatches was calculated for each hen. A multicode was created combining generations (2 generations), houses (3 houses), and tierbatteries where the hens were allocated (4 batteries with 3 tiers per house). The genetic parameters for the average F, HoS and HoF were estimated based on the following linear multi-trait animal model:y = Xb + Za + e
Where: y = vector of observations on t traits; b = vector of fixed effects of the multicode (year, house and battery/tier); a = vector of random additive genetic effects; e = vector of random errors; X and Z = known design matrix of fixed effects and random additive effects, respectively. Variance and covariance components were estimated using the REML-method of the software VCE4 (Neumeier and Groeneveld, 1998). Although the distribution of reproductive traits is not normal, the percentage data of hatchability and fertility were not converted to arcsine, because the benefit of transformation is relatively small (Förster et al., 1993). Moreover, the breeding values on a transformed scale have no biological meaning and are difficult to interpret (Savas et al., 1999). Breeding values were calculated by adding the line mean to the BLUP values estimated with the software PEST (Groeneveld et al., 1990).
Results and discussion
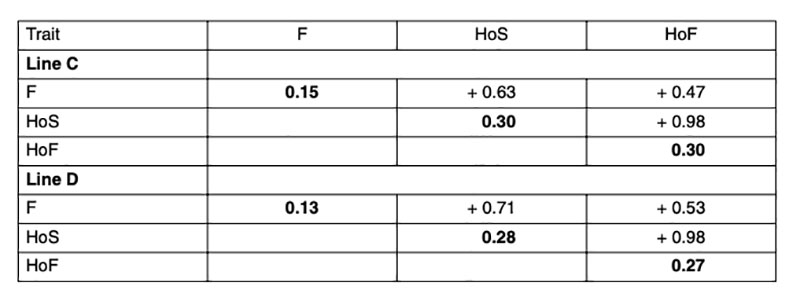
Heritabilities and genetic correlations using model 1 are shown in Table 2. As expected, the estimated heritabilities in this study were higher for hatchability compared to fertility, which is in accordance with other studies (Förster, 1993; Szwaczkowski et al., 2000; Bennewitz et al., 2007). On the contrary, other authors have found lower heritabilities for hatchability compared to fertility (Beaumont et al., 1997; Hartmann et al., 2002; Sharifi et al., 2010; Wolc et al., 2010). High genetic correlations were found between the reproductive traits (F, HoS and HoF), as has been consistently reported in the literature. That means that hens laying a high proportion of fertile eggs also tend to have a high hatchability. Since the genetic correlation between HoF and HoS is very close to one, it would be sufficient to use one of these traits or total number of chicks during the hatching egg saving period as basis for selection.Table 2: Heritabilities (diagonal) and genetic correlations for fertility rate (F), hatch of eggs
set (HoS) and hatch of fertile eggs (HoF)
The estimated heritabilities were similar for both lines and in the case of hatchability slightly higher than values reported in the literature, where the heritability ranged from 0.02 to 0.24 by applying different statistical methods (Förster, 1993; Beaumont et al., 1997; Szwaczkowski et al., 2000; Sapp et al., 2004; Bennewitz et al. 2007, Rozempolska et al., 2009, Sharifi et al., 2010; Wolc et al., 2010). Estimates from different studies in the literature are difficult to compare, due to different definition of traits, data collection and structure of the data and statistical models used in the analysis. Using a cumulative model may overestimate the heritability (Sapp et al., 2004; Swalve, 1995). Higher heritability and accuracy of selection can be obtained by averaging fertility over several weeks, either by pooling all weeks or by calculating average fertility (Wolc et al., 2009).
Genetic correlations were estimated between reproductive traits and other economically important traits, including:
- Egg Production: at the start of the laying cycle (P1), around peak production (P2) and at the end of the laying cycle (P3).
- Egg Quality: Egg Weight (EW), Shell Strength (SS), Dynamic Stiffness (DS), Egg Shape (ES, length/width), Albumen Height (AH), Yolk Proportion (YP).
- Egg Quality: Egg Weight (EW), Shell Strength (SS), Dynamic Stiffness (DS), Egg Shape (ES, length/width), Albumen Height (AH), Yolk Proportion (YP).
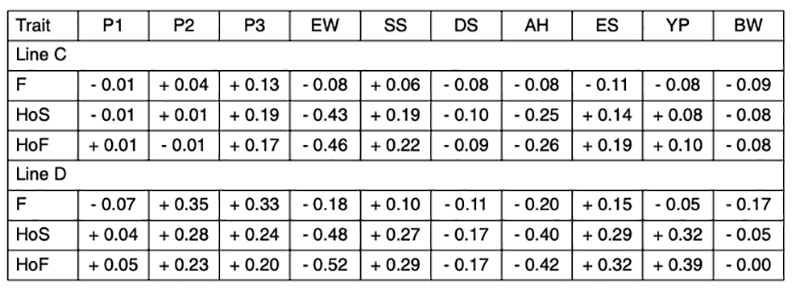
Since the genetic correlations between fertility and different egg quality and production traits are generally low (Table 3), and no distinction could be made between true infertility and early embryonic mortality, we will focus on the genetic correlations involving hatch of fertile eggs (HoF).
Table 3: Estimated genetic correlations between reproductive traits and production and egg
quality traits
The negative correlation between egg weight and hatchability (rg = -0.46 and -0.52) in both lines (Table 3) confirms earlier results of Förster et al. (1992), who found correlations ranging from rg = -0.50 to -0.54 in two brown-egg pure lines. In a breeding program for layers the male lines can be selected for higher egg weight if the female lines lose egg weight as a result of more emphasis on reproductive traits. In this way, it is possible to overcome the negative correlations and maintain a balanced performance profile in the commercial layers. Heterosis effects are also utilized for fitness traits (Förster et al., 1992).
Consumers in Europe prefer eggs of size M or L, i.e. between 53 and 73 g, but the average egg size would be under this economical optimum if this trait were not selected for, due to negative correlations with fitness traits (Stöve-Schimmelpfening and Flock, 1982). Large eggs tend to have relatively less shell surface, and that can be an obstacle for normal gas exchange for the embryo (Narushin and Romanov, 2002).
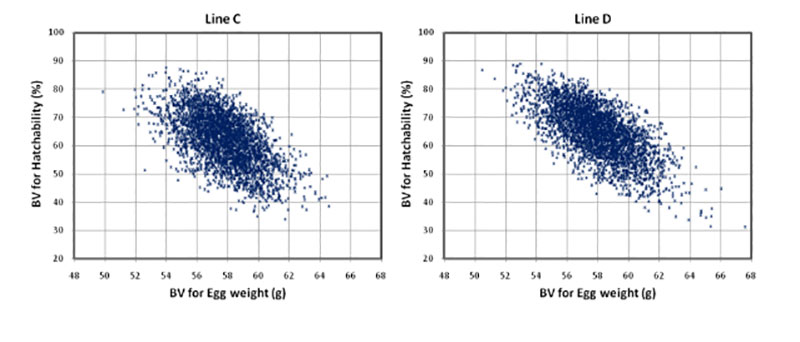
The higher negative correlation between egg weight and hatchability of fertile eggs (HoF) compared to hatchability of eggs set (HoS) and Fertility (FER) suggests that higher egg weight might mainly affect late embryonic mortality and may prolong incubation time, which is in accordance with practical experience. Egg weight at start of incubation seems to have an effect on the time of hatch within the hatch window, thus, early hatchers had a significant lower starting egg weight as compared to late hatchers (Careghi et al., 2005). Therefore in this study, where the incubation time was shorted, eggs with a high egg weight might be additionally penalised in hatchability results. It has been suggested that it may be a nonlinear relationship between egg weight and hatchability (Wolc et al., 2010). As shown in Figure 1, that is not the case in this study, thus a clear linear negative effect of egg weight can be distinguished.
Figure 1: Breeding values for hatch of fertile eggs (HoF) plotted against breeding values for
egg weight
A positive correlation was found between egg production at the end of the laying period and hatchability and fertility, especially in line D, which was also shown during peak production. The values are according to the values indicated by Förster et al. (1993), who gave a possible explanation based on the negative correlation existing between egg production and egg weight. This author further argued that the first egg in a sequence shows a lower hatchability, and that hens with lower productivity have a higher number of first eggs. Förster et al. (1992) reported a decrease in hatchability ranging from 4 to 9% comparing first egg and middle egg in a sequence, this drop was even bigger for single egg sequences (12-14%). Robinson et al. (1991) found that the eggs in the first position of the laying series had lower fertility compared to the following eggs. However, other authors did not find significant differences in hatchability relative to time of oviposition (Zakaria et al., 2005), where early laid eggs were associated to first-in-sequence eggs.
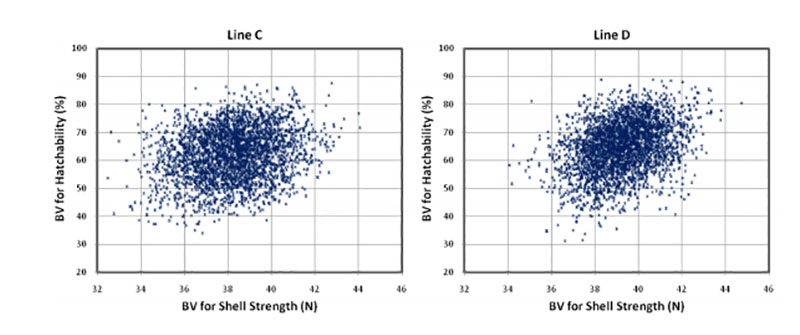
The eggshell performs a double function during embryo development. It has to be strong enough to protect the embryo from external influences and penetration of pathogens, but at the same time it should have enough porosity to allow gas exchange for embryonic development (Narushin and Romanov, 2002). These authors also indicated that hatchability tends to increase with increasing shell thickness and length-to-width ratio. The positive effect of egg shell quality on hatchability has been confirmed in several studies (i.e. Bennet, 1992; Barnett et al., 2004). The correlations estimated in our study range from rg = +0.19 to +0.29 (Table 3), which is in agreement with the positive genetic correlation reported by Wolc et al. (2010) between specific gravity and hatchability (rg = + 0.53). The relationship between shell strength and hatchability is plotted in Figure 2.
Figure 2: Breeding values for hatch of fertile eggs (HoF) plotted against breeding values for
shell strength
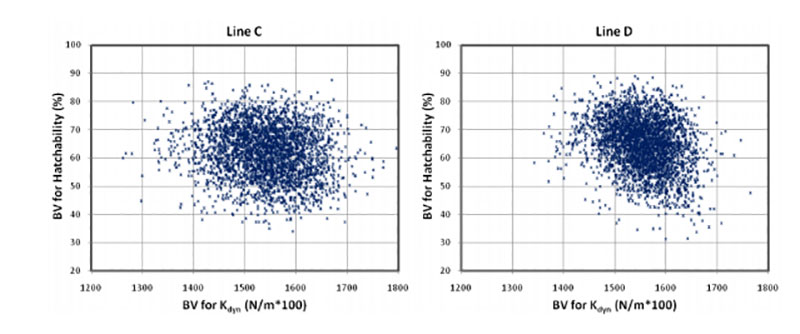
Coucke et al. (1999) proposed that acoustic resonance frequency analysis could measure the mechanical stiffness of intact eggs and defined a novel eggshell parameter, dynamic stiffness (Kdyn). De Ketelaere et al. (2003) propose that Kdyn might provide a better indicator of an egg’s ability to withstand insult because most forces leading to breakage are dynamic and not static. Dunn et al. (2005) and Icken et al. (2006) found that Kdyn has a higher heritability than breaking strength, while the correlation between these traits was significantly below 1 (rg = +0.49 and +0.40). This suggests that Kdyn measures different aspects of the mechanical properties of the egg, in particular structural strength (Dunn et al., 2005). Although the negative genetic correlations with hatchability are low (rg = -0.09 and -0.17) strong selection on Kdyn may not help to improve hatchability, especially in line D, as shown in Figure 3.
Figure 3: Breeding values for hatch of fertile eggs (HoF) plotted against breeding values for
dynamic stiffness (Kdyn)
A possible explanation is that Kdyn has a lower negative correlation with egg weight than breaking strength (Dunn et al., 2005; Icken et al., 2006). A negative effect of increased dynamic stiffness may also be the result of reduced shell porosity, which is essential for gas exchange and for embryo development. The number and size of pores influences the rate of moisture loss and heat conductance across the eggshell (Hulet et al., 2007). Further research is desirable to substantiate this tentative explanation.
The genetic correlation between albumen height and hatchability (rg = -0.26 and -0.42) would perhaps have been less highly negative, if albumen height had been transformed to Haugh Units to remove the effect of the egg weight. Nevertheless, the negative correlation between albumen quality and hatchability is real (Flock et al., 2007) and may be due to limited nutrient availability in eggs with higher albumen and lower yolk percentage. Wolc et al. (2010) reported also a negative correlation between Haugh Units and hatchability (rg = -0.25). Practical experience has also shown that the hatchability of very fresh eggs, which have high Haugh Units, is lower than after a few days of storage (Förster et al., 1992), but that is not relevant here. Albumen consistency is mainly an issue in connection with storage conditions for shell eggs in retail stores rather than for hatcheries.
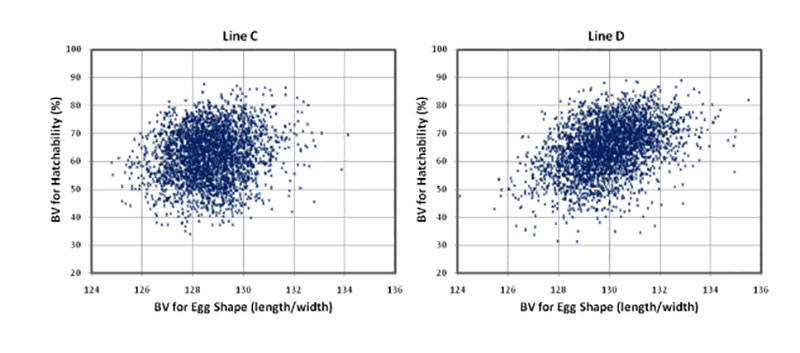
Round eggs often have lower hatchability. The positive correlations between egg shape (length/with) and hatchability (rg = +0.19 and +0.32) confirm that “longer” eggs tend to hatch better. Most eggs used in this study had shapes within the normal range, and eggs with abnormal shapes were already sorted out before setting.
Figure 4: Breeding values for hatchability of fertile eggs (HoF) plotted against the breeding
values for egg shape
A high proportion of yolk, i.e. a high dry matter content of the egg, is appreciated by the egg-processing industry. The breeding goal is therefore at least 30% yolk in these white egg lines (Flock et al., 2007), not only for the egg-processing industry, but also for optimal hatchability and chick quality. We confirmed positive correlations with hatchability (rg = +0.32 and +0.39 for HoS and HoF, respectively). Similar results in a White Leghorn line were also reported by Hartmann et al. (2002), who found positive correlations between hatch of fertile eggs and yolk weight, yolk proportion and albumen dry matter of rg = +0.28, +0.52 and +0.26, respectively. Narushin and Romanov (2002) concluded from the values obtained in the literature that hatchability decreased when the liquid content of the egg increased. Milisits et al. (2010) used electrical conductivity measurements to demonstrate that the hatchability of eggs benefits from a high egg yolk ratio.
In our study, this relation can be partly explained by the negative correlation between yolk proportion and egg weight (rg = -0.60 and -0.65). The composition of the egg changes with egg size: yolk proportion decreases with increasing egg weight. This decrease in yolk proportion may have a negative effect on the nutrient supply of the embryo and consequently on the hatchability of larger eggs (Förster et al., 1992). Wilson (1997) emphasized the crucial role of maternal nutrients for the developing avian embryo and concluded that inadequate, excessive or imbalanced levels of nutrients could even have lethal effects.
In contrast to Förster et al. (1993), who reported negative correlations in two brown layer lines ranging from rg = -0.12 to -0.25, no significant correlation was found between body weight and hatchability in these White Leghorn lines.
Conclusions
Predictable hatchability of first quality chicks within a narrow time window is a common objective for commercial hatcheries. To improve reproductive traits by genetic selection, they must be included in the selection index, with proper attention to all genetic correlations to other traits. In view of the negative correlation with egg weight, focus on hatchability should be limited to female lines, while selection for desirable egg weight is practiced in male lines. With this strategy, egg weight could decline in the female lines toward a level determined by chick quality standards. A possibly negative relationship between dynamic stiffness of egg shells and hatchability can also be taken care of with appropriate choice of shell quality criteria in male and female lines. Persistent egg production and high yolk percentage are positively related to hatchability, and only the negative correlation with albumen height indicates a conflict with traditional breeding goals in White Leghorns.References
BAMELIS, F. (2003): Non invasive assessment of eggshell conductance and different developmental stages during incubation of eggs. Ph.D. Thesis, KU Leuven, Belgium.BARNETT, D.M., B.L. KUMPULA, R.L. PETRYK, N.A. ROBINSON, R.A. RENEMA and F.E. ROBINSON (2004): Hatchability and early chick growth potential of broiler breeder eggs with hairline cracks. Journal of Applied Poultry Research, 13: 65-70.
BEAUMONT, C., N. MILLET, E. LE BIHAN-DUVAL, A. KIPI and V. DUPUY (1997): Genetic parameters of survival to the different stages of embryonic death in laying hens. Poultry Science, 76: 1193-1196.
BENNET, C. D. (1992): The influence of shell thickness on hatchability in commercial broiler breeder flocks. Journal of Applied Poultry Research, 1: 61-65.
BENNEWITZ, J., O. MORGADES, R. PREISINGER, G. THALLER and E. KALM (2007): Variance component and breeding value estimation for reproductive traits in laying hens using a Bayesian threshold model. Poultry Science, 86: 823-828.
CAREGHI, C., K. TONA, O. ONAGBESAN, J. BUYSE, E. DECUYPERE and V. BRUGGEMAN (2005): The effects of spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of storage. Poultry Science, 84: 1314-1320.
COUCKE, P., E. DEWIL, E. DECUYPERE and J. BAERDEMAEKER (1999): Measuring the mechanical stiffness of an eggshell using resonant frequency analysis. British Poultry Science, 40: 227-232.
DE KETELAERE, B., H. VANHOUTTE and J. DE BAERDEMAEKER (2003): Parameter estimation and multivariable model building for the non-destructive, on-line determination of the eggshell strength. Journal of Sound and Vibration, 266: 699- 709.
DECUYPERE E. and V. BRUGGEMAN (2007): The endocrine interface of environmental and egg factors affecting chick quality. Poultry Science, 86: 1037-1042.
DUNN, I.C., M. BAIN, A. EDMOND, P.W. WILSON, N. JOSEPH, S. SOLOMON, B. DE KETELAERE, J. DE BAERDEMAEKER, M. SCHMUTZ, R. PREISINGER and D. WADDINGTON (2005): Heritability and genetic correlation of measurements derived from acoustic resonance frequency analysis; a novel method of determining eggshell quality in domestic hens. British Poultry Science, 46: 280-286.
FLOCK, D.K. (1995) Changing emphasis in genetic development of egg-type chickens. Archiv für Tierzucht 47: 479-488.
FLOCK, D.K., M. SCHMUTZ and R. PREISINGER (2007): Optimierung der Eiqualität aus züchterischer Sicht. Züchtungskunde, 79: 309-319.
FÖRSTER, A., E. KALM and D.K. FLOCK (1992): Schlupffähigkeit aus züchterischer Sicht. Lohmann Information (Sept./Okt.): 13-16.
FÖRSTER, A. (1993): Züchterische Möglichkeiten einer Verbesserung der Schlupfrate in Reinzuchtlinien eines Zuchtprogrammes für braune Legehybriden. PhD Thesis. Schriftenr. Inst. Tierzucht und Tierhaltung, Christian-AlbrechtsUniv., Kiel, Germany.
FÖRSTER, A. (1994): Optimierung der Schlupfrate und Kükenqualität – biologische Zusammenhänge und Bruttechnik. Lohmann Information (Mai/August): 11-16.
GROENEVELD, E., KOVAC, M., WANG, T. (1990): PEST, a general purpose BLUP package for multivariate prediction and estimation. Proceedings of the 4th World Congress on Genetics Applied to Livestock Production, Edinburgh, UK. Pages: 488-491.
HARTMANN, C., E. STRANDBERG, L. RYDHMER and K. JOHANSSON (2002): Genetic relations between reproduction, chick weight and maternal egg composition in a white leghorn line. Acta Agriculturae Scandinavica, Sect. A, Animal Science 52: 91-101.
HULET, R., G. GLADVS, D. HILL, R. MEIJERHOF and T. EL-SHIEKH (2007): Influence of egg shell embryonic incubation temperature and broiler breeder flock age on posthatch growth performance and carcass characteristics. Poultry Science, 86: 408-412.
ICKEN, W., M. SCHMUTZ and R. PREISINGER (2006): Dynamic stiffness measurements with the “crack detector”: a new method to improve egg shell strength. Lohmann Information, 41 (1): 13-19.
MAYES, F.J and M.A. TAKEBALLI (1984): Storage of the eggs of the fowl (Gallus domesticus) before incubation: a review. World’s Poultry Science Journal, 40: 131-140.
MEIJERHOF, R. (1992): Pre-incubation holding of hatching eggs. World’s Poultry Science Journal, 48: 58-67.
MILISITS G., E. KOVÁCS, O. POCZE, J. UJVÁRI, Z.S. TARASZENKÓ, G. JEKKEL, L. LOCSMÁNDI, GY. BÁZÁR, A. SZABÓ, R. ROMVÁRI, and Z SÜTO (2010): Effects of egg composition on hatchability and on growth and slaughter characteristics of meat-type chicks. British Poultry Science 51: 289-295.
NARUSHIN, U.G. and M.N. ROMANOV (2002): Egg physical characteristics and hatchability. World’s Poultry Science Journal, 58: 297-303.
NEUMAIER, A. and E. GROENEVELD (1998): Restricted maximum likelihood estimation of covariances in sparse linears model. Genetics Selection Evolution, 30: 3-26.
ROBINSON, F.E., R.T. HARDIN, N.A. ROBINSON and B.J. WILLIAMS (1991): The influence of egg sequence position on fertility, embryo viability, and embryo weight in broiler breeders. Poultry Science, 70: 760-765.
ROZEMPOLSKA-RUCINSKA I., G. ZIEBA and M. LUKASZEWICZ (2009): Hatchability traits as selection criteria in breeding of laying hens. Archiv für Geflügelkunde, 73: 263-267.
SAVAS, T., R. PREISINGER, R. RÖHE and E. KALM (1999): Einfluss der Box-Cox Transformation auf die Schätzung genetischer Parameter für Legerate bei Legehennen. Züchtungskunde, 71: 380-391.
SAPP, R.L., R. REKAYA, I. MISZTAL and T. WING (2004): Male and Female Fertility and Hatchability in Chickens: A Longitudinal Mixed Model Approach. Poultry Science, 83: 1253-1259.
SHARIFI, A.R., R. PREISINGER, F. VAHIDI, K. JAWASREH and H. SIMIANER (2010): Statistical and genetic analysis of reproductive traits in laying hens. Proceedings of the 9th World Congress on Genetics Applied to Livestock Production, Leipzig, Germany.
STOVE-SCHIMMELPFENNIG, K. and D.K. FLOCK (1982): Eigewicht und relative Fitness in der Legehennenzüchtung. Archiv für Geflügelkunde, 46: 270 – 275.
SWALVE, H.H. (1995): The effect of test day models on the estimation of genetic parameters and breeding values for dairy yield traits. Journal of Dairy Science, 78: 929-938.
SZWACZKOWSKI, T., S. WEZYK, P. PIOTROWSKI and K. CYWA-BENKO (2000): Direct and maternal genetic and environmental effects for fertility and hatchability in laying hens. Archiv für Geflügelkunde, 64: 115-120.
TONA, K., F. BAMELIS, B. DE KETELAERE, V. BRUGGEMAN, V. M. B. MORAES, J. BUYSE, O. ONAGBESAN AND E. DECUYPERE (2003): Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poultry Science, 82: 736-741.
WILLEMSEN, H., N. EVERAERT, A. WITTERS, L. DE SMIT, M. DEBONNE, F. VERSCHUERE, P. GARAIN, D. BERCKMANS, E. DECUYPERE AND V. BRUGGEMAN (2008): Critical Assessment of Chick Quality Measurements as an indicator of posthatch Performance. Poultry Science, 87: 2358-2366.
WILSON, H.R. (1991): Interrelationships of egg size, chick size, posthatching growth and hatchability. World’s Poultry Science Journal 47: 5-20.
WILSON, H.R. (1997): Effects of maternal nutrition on hatchability. Poultry Science, 76:134-143.
WOLC, A., I.M.S. WHITE, V.E. OLORI and W.G. HILL (2009): Inheritance of fertility in broiler chickens. Genetic Selection Evolution, 41:47.
WOLC, A., I.M.S. WHITE, W.G. HILL and V.E. OLORI (2010): Inheritance of hatchability in broiler chickens and its relationship to egg quality traits. Poultry Science, 89: 2334-2340.
ZAKARIA A.H., P.W. PLUMSTEAD, H. ROMERO-SANCHEZ, N. LEKSRISOMPONG, J. OSBORNE AND J. BRAKE (2005). Oviposition pattern, egg weight, fertility and hatchability of young and old broiler breeders. Poultry Science, 84: 1505- 1509.
Zusammenfassung
Züchterische Verbesserung der Schlupfrate bei Weißen Leghorn (LSL)Pedigree-Daten von zwei LSL Reinzuchtlinien wurden ausgewertet, um genetische Parameter für Fruchtbarkeit und Schlupfrate und Korrelationen zu Eiqualitätskriterien zu bestimmen. Die Schlupfrate befruchteter Eier hatte mit 0,27-0,30 eine deutlich höhere Heritabilität als die Fruchtbarkeit mit 0,13-0,15 und ist deshalb als Selektionsmerkmal geeigneter. Genetische Korrelationen zum Eigewicht und zum Eiklaranteil waren deutlich negativ, während positive Beziehungen zum Dotteranteil und zur Schalenstabilität bestehen. Daraus ergibt sich die Empfehlung, bei Hennenlinien auf höheren Dotteranteil zu selektieren und eine Reduzierung des Eigewichts durch natürliche Selektion in Kauf zu nehmen. Ein marktgerechtes Eigewicht kann durch entsprechende Selektion auf Eigewicht in den Hahnenlinien abgesichert werden.