Abstract
Feather pecking and mortality due to cannibalism in laying hens are of economic and welfare concern in all housing systems. Traditional family selection on the basis of liveability and feather condition in group cages has reduced, but not eliminated this misbehaviour. Actually, the change to non-cage housing systems and the ban on beak treatment in several countries have increased the risk of feather pecking for egg producers. Primary breeders have tried to find novel selection criteria which can be determined under commercial conditions on a large number of birds. Lohmann Tierzucht started several years ago to measure beak shape in pedigreed hens of different lines and estimated genetic correlations with feather cover. In group cages, birds with shorter and blunt beaks tended to have better feather quality and lower mortality. However, the correlations vary considerably between lines (rg = -0.30 to +0.05 for feather quality and -0.08 to +0.19 for mortality). We conclude from our results that individual selection for blunt beaks in addition to family selection for intact feather cover and liveability may accelerate the reduction of feather pecking and cannibalism, while efforts continue to optimize management practices.
Keywords
laying hens, animal welfare, feather pecking, beak shapeCo-Authors
D. Cavero and M. Schmutz
Introduction
During recent decades, the social behaviour and welfare of laying hens have received more attention in laying hen breeding programs. However, the great variability of behaviours makes it difficult to define what is disturbed behaviour and negatively affecting the wellbeing of other birds in a group. Only damaging behaviours such as feather pecking and cannibalism are unanimously recognized as disturbed behaviour (Bessei, 2016). Feather pecking is of special concern, and this welfare issue is getting more attention since beak treatment will be banned in many countries in the near future (Rodenburg et al., 2013).Different theories have been proposed to explain the behavioural cause of feather pecking. The most commonly accepted one is based on the two main components of foraging behaviour in birds: food searching (the appetitive phase) and food consumption (the consummatory phase). Keeling (2002) argued, that feeding ad libitum may satisfy the motivation for the consummatory phase, however the birds will still exhibit food searching behaviours, such as ground pecking and scratching, which might be redirected in severe feather pecking (Blockhuis, 1986). De Haas et al. (2014) found that the provision of pecking blocks, good quality litter and an enriched environment on commercial farms resulted in less feather damage.
Regardless what the real origin of this behaviour might be, the fact is, that feather pecking is affected by many different factors and although many of them have been identified and intensively studied, it remains largely unpredictable and difficult to control (Hartcher et al., 2016). Therefore a multifactorial approach attending to different parameters should be used to minimize its negative impact. This misbehaviour can occur in every housing system; however it is especially relevant and more variable in cage-free housing systems, due to larger groups of birds and a more complex environment. Feather pecking and cannibalism was recently reviewed in an invited lecture during the World Poultry Congress in Beijing by Bessei (2016), which is highly recommended as background for the present paper.
Daigle et al. (2015) described three different pecking behaviour levels: (i) gentle feather pecking (which does not result in the removal of a feather); (ii) severe feather pecking (which leads to feather losses at the back, rump or tail of the victim); and (iii) aggressive pecking (the most serious type of feather pecking, usually directed at the head and often associated with a chase, standoff, or leap from the aggressor). The last two types are the most relevant from a bird welfare point of view. One of the approaches to reduce this problem is to select directly or indirectly against this antagonistic behaviour.
Feather eating has been associated with feather pecking (Haarlander-Matauscheck and Häusler, 2008; Bennewitz et al., 2014). It has also been suggested that there may be “primary feather-peckers” in a flock with a genetic predisposition to feather pecking due to an increased appetite for feathers and fibre as a consequence of their intestinal microbiota (Meyer et al., 2013; Bessei and Kjaer, 2015). Therefore, it could be used as an indirect trait to select against feather pecking. Hen specific data on “feather eating” was recorded by McKeegan and Savory (2001) and related to feather pecking. In their study, peckers ate, picked up and manipulated feathers significantly more often than non-peckers. However, in commercial group housing, Riber and Hinrichsen (2016) found no correlation between the prevalence of poor plumage condition and the prevalence of droppings with feather content. These authors leave the question unanswered from where feathers are selected for ingestion. Remaining feathers in droppings could be the result of eaten feathers which were picked from the floor litter, plucked directly from other hens or dislodged during preening of own feathers. Thus, data on feathers found in droppings is not a reliable indicator for feather pecking and therefore does not seem useful as a selection trait to improve plumage condition and reduce feather pecking.
Traits related to the incidence of feather pecking such as plumage condition or mortality due to cannibalism recorded from relatives (full and half sibs) in group cages, not only housed in the breeding farms, but also under field conditions in commercial farms has been already included into the selection index of Lohmann layers since decades. The heritability of plumage conditions has a moderate heritability (h² = 0.15-0.30). This selection has allowed reducing the incidence of feather pecking to a low level. However, due to the multifactorial nature of this misbehaviour, it will be difficult to eliminate it completely.
Bennewitz et al. (2014) analyzed individual behaviour data from 910 hens in pens with 36 to 42 birds, collected by seven experienced observers and estimated a heritability of h² = 0.10 for feather pecking and aggressive pecking, which were positively correlated (+0.81). Unfortunately, the results indicated a negative genetic correlation between feather pecking and egg production (rg = -0.5). The automatic direct observation and evaluation of individual bird behaviour in a small group is a technical challenge, and it is extremely time consuming to do it manually.
Kjaer (2009) observed a positive relationship between the locomotor activity of layers and their pecking behaviour from which activity characteristics such as the free range behaviour and nest acceptance were derived. Hen specific data recording on these traits supports a balanced breeding for layers with calm social behaviour and sufficient locomotor activity to move adequately in group housing.
Different management strategies can be applied to reduce the incidence of feather pecking and cannibalism. A widely used method that results in fewer nonagonistic pecks is beak treatment of chicks in the hatchery or before 10 days of age. Various publications describe the pros and cons of different beak trimming methods, however all variations are beneficial in reducing beak-inflicted feather loss and mortality from cannibalistic pecking in commercial flocks. According to Damme and Urselmans (2013) the goal of any beak treatment must be to destroy as little nerve tissue as possible and to induce quick recovery, while reducing the frequency of aggressive pecking. The treatment should minimize pain and interfere as little as possible with feed selection, feed intake and preening, but prevent the misuse of the beak as a pair of tweezers to pull feathers of other hens. The goal is a round beak without sharp or pointed ends, which can be obtained by adequate infrared treatment. Although this beak treatment has been proven to be very effective in preventing feather pecking, ethicists consider this practice as an amputation. Some countries have banned completely this practice and others will join this initiative soon. Banning of beak treatment is a new driving force to find solutions to reduce the incidence of feather pecking.
Pecking stones are being used in commercial farms for layers and turkeys. Abrasive materials for beak blunting may be an effective alternative to beak treatment. In a recent review, van der Linde (2016) noticed that the continuous use of pecking stones in turkeys blunt the beak, so that after 3 to 6 months around 80% of the birds showed no difference between birds with and without beak treatment. Morrissey et al. (2017) also reported that the use of cuttlebones (but not blunting boards) showed encouraging results for shortening upper mandibles in laying hens. Parallel to these management practices, primary breeding companies could select pedigree birds on beak shape under commercial management conditions, as a long-term program with cumulative effects. To what extent genetic selection on beak shape can contribute to this goal will be discussed on the basis of experimental results from pedigreed brown-egg and white-egg layers.
Measurement of beak shape
A special device was developed to measure beak shape in terms of the extension of the upper beak beyond the lower beak in pedigreed hens and to evaluate the usefulness of this criterion as an additional selection criterion to reduce feather pecking. The working hypothesis was that birds with blunt beaks should be less inclined or less successful in pulling feathers from group mates.With this equipment, the excess length of the upper beak compared to the lower beak is measured and automatically saved in a database (Fig. 1). Three generations of objective and reliable data on beak shape from Lohmann pure line layers were available for this study. Since the beaks not only continue to grow, but at the same time respond to abrasion in the given environment, the age at the time of measurement has to be standardized to compare individual hens in a group.

Figure 1: Measurement of beak shape in terms of the difference between upper and lower beak
As shown in Table 1, average excess of the upper beak was similar at different ages and for different brown egg lines. Apparently the growth of the beak tissue compensated or even exceeded the abrasion in single hen cages. The tested hens were housed in modified single bird cages that include perches, a scratching area as well as a special claw abrasion board. Drinking nipples and a commercial feed chain ensure the ad libitum supply of nutrients for the layers. Line D has a shorter beak, whereas differences between the other lines are marginal. The phenotypic correlations between measurements at 23 and 48 weeks of age within line vary between rp= +0.30 and rp= +0.42, indicating satisfactory repeatability of measurements at different ages. Thus, beak shape appears to be a promising trait for selection against feather pecking, as long as it is recorded at the same age.
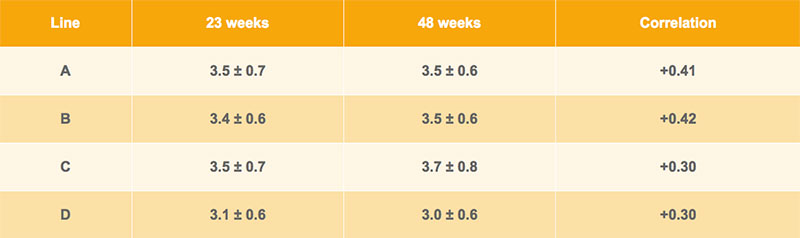
Table 1: Averages ± standard deviations for beak length (mm) at 23 and 48 weeks of age and their phenotypic correlation for Lohmann Brown pure lines
Brown-egg and white-egg pure-lines are compared in table 2. These averages are based on about 3,000 individual hens per line, measured at about 30 weeks of age. As in table 1, the beaks in the brown-egg line D are slightly shorter. However, differences between brown and white layers are larger. At the same age, the differences between upper and lower beak of LSL layers are 0.4 to 0.8 mm shorter compared to the lines of Lohmann Brown. Individual variation within line in difference of beak is substantial; the variation coefficient is more than 20.
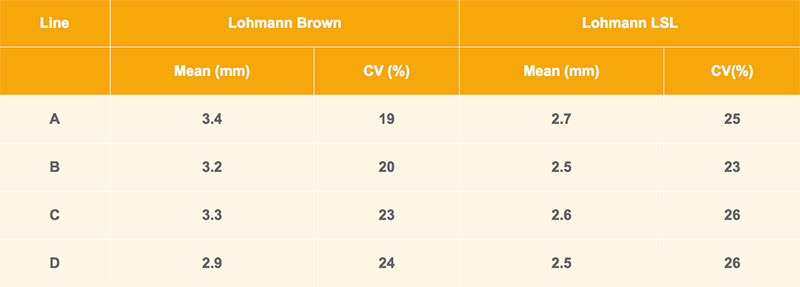
Table 2: Comparison of beak shape in brownegg vs. white-egg lines at 30 weeks of age: means with coefficient of variation (CV)
The trait follows a normal distribution, as shown in Fig. 2 for one line. The results in tables 3 and 4 show to what extent this variation is genetically determined.
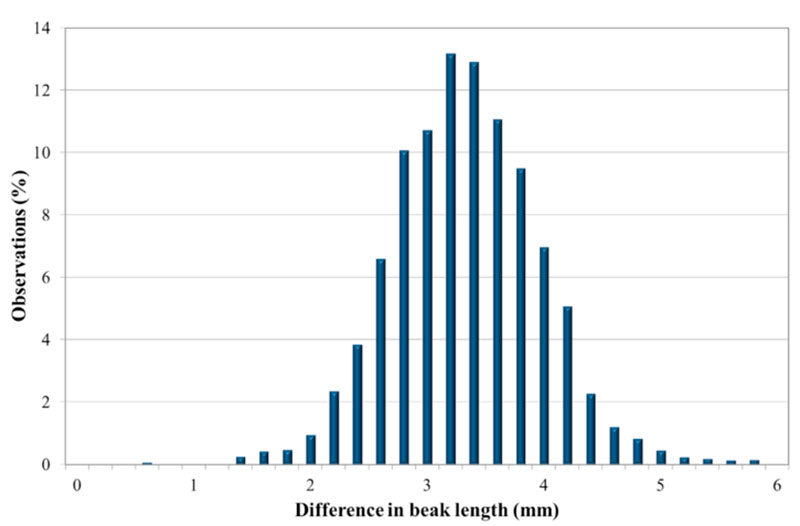
Figure 2: Distribution of the difference of beak length for Lohmann Brown — Line A.
The heritability estimates for beak shape range from 0.13 to 0.25 and from 0.09 to 0.26 for four lines each of the Lohmann Brown and LSL breeding programs, respectively. These genetic parameters and the high variability of the trait suggest that a reduction of beak length through genetic selection should be feasible. The heritabilities are at a similar level as for traits like plumage condition or persistency of egg production. Before including a new trait in a selection index for commercial lines, genetic correlations with other important traits must be analysed and taken into account.
Genetic correlations between beak shape and the egg number at different production levels are shown in tables 3 and 4 for Lohmann Brown and LSL respectively. In three out of four brown-egg lines, hens with shorter upper beak tended to lay more eggs, whereas a longer upper beak was positively correlated with egg production in the white-egg lines (table 4). However, most of the correlations are nonsignificant and the differences between lines are difficult to explain.
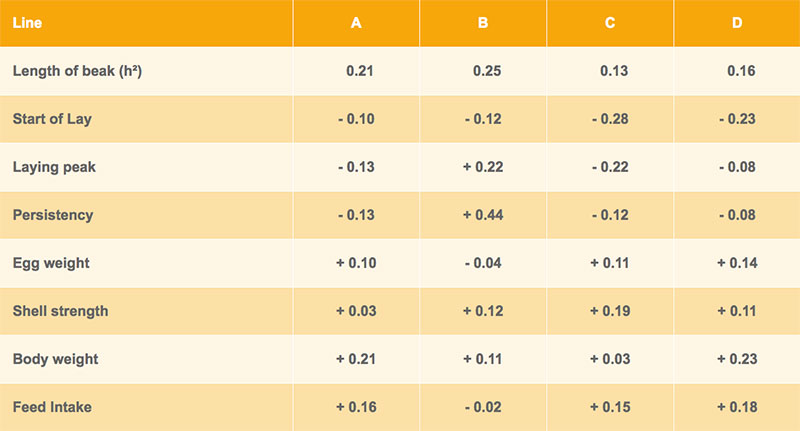
Table 3: Heritability of beak shape and genetic correlations with other traits – Lohmann Brown
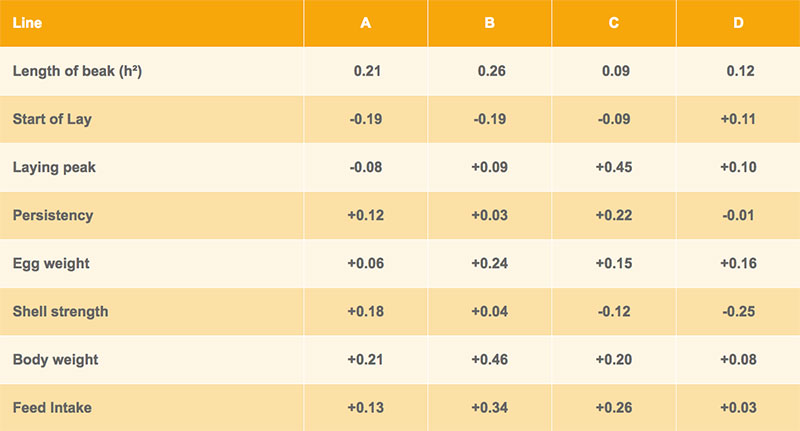
Table 4: Heritability of beak shape and genetic correlations with other traits – Lohmann LSL
Pedigreed hens of the commercial Lohmann Brown and LSL lines have been scored for their plumage condition since more than twenty years, based full-sibs and half-sibs housed in group cages, both in the breeding farms as well as in commercial farms under field conditions.
In the field tests, the layers are scored for their plumage condition at about 40 and 75 weeks of age. Families with intact plumage are getting the top score 9, families with damaged feathering are downgraded according to the amount of feather loss. Based on this information, genetic correlations were estimated between beak length and plumage condition and mortality. As shown in table 5, the correlation between mortality and beak length is mostly a positive, the correlation between beak length and plumage condition mostly negative. Although the standard errors of the estimates are high, birds with shorter beaks tend to have lower mortality and better plumage condition, which supports our initial working hypothesis, especially in the white egg layers (Lohmann LSL).
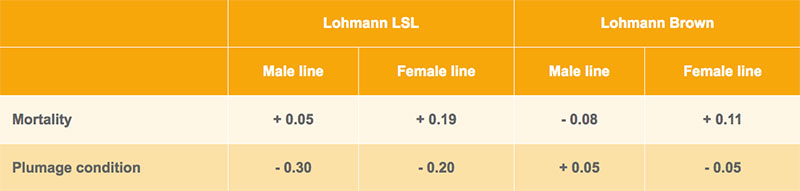
Table 5: Genetic correlations between beak shape and plumage condition and mortality in group cages in commercial farms under field conditions
Discussion
For students of evolution, the different beak shapes of finches on the Galapagos Islands are a familiar model to support Darwin’s theory of evolution in response to a changing environment. Weiner (1984) quoted more recent observations on these finches as evidence that evolution continues and is measurable in terms of changing bird populations in response to “dry” and “wet” seasons. The beak of chickens evolved as a useful tool to search for food in a free range environment, and this tool is misused by some birds as a “weapon” for aggressive pecking and/or cannibalism. Beak treatment of chicks at an early age had been used successfully in the poultry industry as a very effective means to minimize the risk of feather pecking and related mortality. The ban on beak treatment forces primary breeders to take a closer look at genetic variation between and within lines in measurable traits which are directly or indirectly related to feather pecking and cannibalism. Measuring the shape of beaks as described in this study has the advantage that it can be applied in large numbers of birds in a non-cage environment.Summary and Conclusions
The reduction of feather pecking of laying hens is recognized as a major challenge for egg producers and primary breeders of laying hens due to its negative association with the wellbeing of the birds. To reduce the incidence, geneticists in the past have relied mainly on family selection for liveability and feather condition in group cages. Beak shape, defined as the excess in length of the upper beak over the lower beak, was measured in pedigreed hens over a period of three generation to estimate genetic parameters, which are reported here. The results suggest that including beak shape as an indicator of feather pecking behaviour in a multi-trait index may accelerate the desired reduction of feather pecking and cannibalism in flocks of laying hens which are not beak treated. However, family selection for intact feather cover and liveability should continue and management practices have to be further improved.

References
BENNEWITZ, J., S. BOEGELEIN, P. STRATZ, M. RODEHUTSCORD, H.P. PIEPHO, J.B. KJAER and W. BESSEI (2014) Genetic parameters for feather pecking and aggressive behaviour in a large F2-cross of laying hens using generalized linear mixed models. Poultry Science 93: 810-817.BESSEI, W. (2016) Impact of animal welfare on worldwide poultry production. Proc. of the XXV World’s Poultry Congress in Beijing 5-9 September – Invited Lecture Papers: 105-110.
BESSEI, W. and J.B. KJAER (2015) Feather pecking in layers – state of research and implications. Proc. Australian Poultry Science Symposium in Sydney 29–11 February, Vol 26: 214-221.
BLOCKHUIS, H.J. (1986) Feather-Pecking in Poultry – Its relation with ground pecking. Applied Animal Behaviour Science 16: 63-67.
DAIGLE, C.L., T.B. RODENBURG, J.E. BOLHUIS, J.C. SWANSON and J.M. SIEGFORD (2015) Individual consistency of feather pecking behaviour in laying hens: once a feather pecker always a feather pecker? Frontiers in Veterinary Science 2 (6): 1-12.
DAMME, K. and S. URSELMANS (2013) Infrared beak treatment – a tempory solution? Lohmann Information 48 (2): 36-44.
DE HAAS, E.N., J.E. BOLHUIS, I.C. DE JONG, B. KEMP, A.M. JANCZAK and T.B. RODENBURG (2014) Predicting feather damage in laying hens during the laying period. Is it the past or is it the present? Applied Animal Behaviour Science 160: 75-85.
Van der Linde, J. (2016). Legehennen mit intakten Schnabel halten – “Wir müssen uns helfen”. DGS Magazin 48: 15-18.
HAARLANDER-MATAUSCHECK A. and HÄUSLER (2008). Understanding feather eating behavior in laying hens. Applied Animal Behavior Science 117: 35-41.
HARTCHER K.A., S.J. WILKINSON, P.H. HEMSWORTH and G.M. CRONIN (2016) Severe feather-pecking in non-cage laying hens and some associated and predisposing factors: a review. World’s Poultry Science Journal 72: 103-114.
KJAER, J.B. (2009) Feather pecking in domestic fowl is genetically related to locomotor activity levels: implications for a hyperactivity disorder model of feather pecking. Behav. Genet. 39 (5): 564-570.
MEYER, B., J. ZENTEK and A. HARLANDERMATAUSCHECK (2013) Differences in intestinal microbial metabolites in laying hens with high and low levels of repetitive feather-pecking behavior. Physiology and Behavior 110: 96-101.
MCKEEGAN, D.E.F. and C.J. SAVORY (2001) Feather eating in in individually caged hens which differ in their propensity to feather peck. Appl. Anim. Behav. Sci. 73: 131-140.
MORRISSEY, K.L.H., T.M. WIDOWSKI, S. BLOCKLERHURST and V. SANDILANDS (2017) Quantifying the effects of cuttlebones and an abrasive beak blunting object on beak shape and pecking force in two breeds of laying hens. Proc. of the Annual Meeting of the WPSA (UK Branch) 26-27 April in Chester: 020.
RIBER, A.B. and L.K. HINRICHSEN (2016) Feather pecking and its associations with plumage damage and feathers on the floor in commercial farms of laying hens. Animal 10 (7): 1218-1224.
RODENBURG, T.B., M.M. VAN KRIMPEN, I.C. DE JONG, E.N. DE HAAS, M.S. KOPS, B.J. RIEDSTRA, R.E. NORDQUIST, J.P. WAGENAAR, M. BESTMAN and C.J. NICOL (2013) The prevention and control of feather pecking in laying hens: identifying the underlying principles. World’s Poultry Science Journal 69: 361-373.
WEINER, J. (1994) Der Schnabel des Finken oder Der kurze Atem der Evolution: Was Darwin noch nicht wusste. Droemer Knaur. German translation from „The beak of the finch. Evolution in Real Time“).