Summary
Salmonella (S.) Infantis infections in humans transmitted by food are increasingly observed around the
world. S. Infantis strains from several origins isolated between 2002 and 2009 were subtyped by
phage typing and macrorestriction analysis (MRA). It was found that the 617 S. Infantis isolates
investigated belonged to 47 defined phage-types and the 255 isolates characterized by MRA to 45
XbaI-types. The combination of these typing methods revealed a great diversity of clones among the
S. Infantis isolates as well as occurrence of identical clones (PT 1/XbaI 34, PT 29/XbaI 27) in chicken
meat and in humans in Germany over several years. Several hospital infections caused by these
above-mentioned clones were also identified. Circulation of clone PT 29/XbaI 5 in broiler flocks
between Hungary und Germany has been demonstrated by complex typing. The development of
country-specific epidemiologic processes was also observed as PT 61 in Iceland. The change of
predominant Salmonella serovars in the ecologic niche “broiler”, vacated by vaccination against
S. Typhimurium and S . Enteritidis, may lead to an epidemic increase of S. Infantis in broiler flocks.
Routine application of the new phage typing scheme can be used to monitor the spread of S. Infantis.
Introduction
Salmonella spp. are considered to be one of the most common causes of food borne bacterial infections. According to WHO estimates, Salmonella (S.) is responsible for 3 million infections of humans annually (Crump et al. 2004). In Germany 31.185 Salmonella infections were reported to the Robert KochInstitute in 2009. Since the aetiology of enteric infections in many cases is not established and only a fraction of the cases is notified, it is assumed that actually about one million people are infected annually in Germany (Tschäpe & Bockemühl 2002).The host-unspecific serovar S. Infantis ranks first among the top ten human isolates of S. enterica in Germany and Europe since 2001 (Galanis et al. 2006). Since the late 1970s this serovar has been increasingly recorded worldwide in countries like Argentina, Australia, Brazil, The Netherlands, Finland, Canada, Hungary, Japan, New Zealand and Russia. S. Infantis is also observed in hospitals, where primarily small children are infected, but also adults, sometimes with septicaemic symptoms and lethal outcome (Hasenson et al. 1995). Of special importance is that S. Infantis infections may persist in hospitals over a long period of time (Pessoa-Silva et al. 2002; Fonseca et al. 2006).
The reservoir for S. Infantis salmonellosis in humans is primarily seen in animal and especially poultry populations. A survey of the European Food Safety Authority (EFSA) indicates that S. Enteritidis and S. Infantis were mainly found in layer and broiler farms in Europe (Table 1). The highest frequency of serovar S. Infantis in broilers was found in Hungary (87 %), followed by Poland (19 %) and the Czech Republic (13 %) (EFSA 2007a; 2007b). In Germany the occurrence of S. Infantis was 3.9 % in layer flocks and 8.9 % in broiler flocks (BfR 2005, 2006; Table 1).
Table 1: Results from EFSA and BfR studies on occurrence of Salmonella spp. in laying and
broiler farms (EFSA 2007a, 2007b; BfR 2005, 2006)
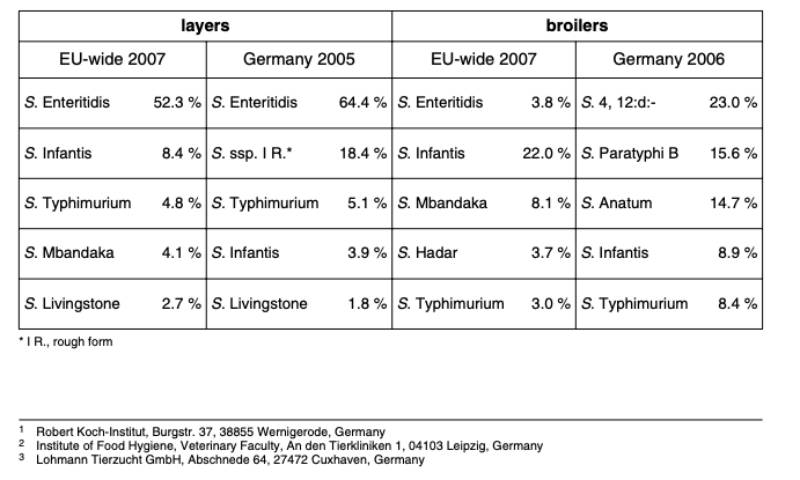
Another study by EFSA on the prevalence of Salmonella in broiler meat showed that S. Infantis was the most frequent serovar in the EU, averaging 55 %. In some countries as Hungary (96 %), Slovenia (55 %) and Austria (44 %) it was the most important serovar (EFSA 2007c). With an incidence of 7 % and 10 % respectively in Europe and Germany, S. Infantis ranked second in eggs and egg products (EFSA 2009).
The persistence of S. Infantis in broiler flocks is not only a problem in Hungary, but also in Japan, Iceland, France, The Netherlands, USA, Australia, Turkey, Saudi-Arabia and Algeria (Ai-Nakhli et al. 1999; Van Duijkeren et al. 2002; Sexton et al. 2007; Thorsteinsdottir et al. 2007; Cetinkaya et al. 2008; Elgroud et al. 2008; Heithoff et al. 2008; Rivoal et al. 2009).
In Germany and several other countries live vaccines against S. Enteritidis and S. Typhimurium are in use. It can be assumed that live vaccination with S. Enteritidis will introduce immunity against Salmonella of group 0:9 (D1) and live vaccination with S. Typhimurium immunity against Salmonella of group 0:4 (B). After successful control of S. Enteritidis and S. Typhimurium apparently other serovars may enter this ecological niche and persist. It is known that S. Enteritidis entered poultry facilities after serovar S. Gallinarum had been eliminated (Rabsch et al. 2000).
S. Infantis of group 0:7 (C1) is a potential candidate for a change of serovars and so far there is no licensed vaccine against S. Infantis. In Hungary and Japan S. Infantis is already the most frequent serovar in broilers, which is mirrored by an increase of human isolates in these countries (Nogrady et al. 2008; Shahada et al. 2008; Ishihara et al. 2009; Noda et al. 2010).
Suitable techniques for typing serovars are required to monitor sources and routes of infections. A method requiring relative little laboratory equipment is phage typing, which uses specific bacteriophages to differentiate strains belonging to the same serovar. Using established phage typing systems for S. Typhimurium, S. Enteritidis and S. Agona it has been possible to document the pathways of infections (Ward et al. 1987; Rabsch et al. 2005; Rabsch 2007). Advantages of phage typing are speed and low cost, but this method requires special expertise to interpret the phage types and strict quality control of the biological reagents (Schwarz et al. 2003).
Material und Methods
In order to monitor the serovar S. Infantis more effectively, a new phage typing system was established at the National Reference Center for Salmonella and other Enteric Pathogens (NRC) of the RobertKoch-Institute. The phage typing follows the standardized NRC-protocol (Rabsch 2007). A total of 617 S. Infantis strains from Germany (n=542), Hungary (n=25), Iceland (n=21), and Australia (n=29) were included in this study and investigated by phage typing. The strains were isolated from outbreaks and sporadic cases of gastroenteritis in humans, from animals, food, feed and the environment during the period 2002 – 2009. For molecular subdifferentiation and examination, 255 out of the 617 S. Infantis isolates were analyzed by macrorestriction analysis after pulsed-field gel electrophoresis using the PulseNet CDC-protocol (Hunter et al. 2005).Results and Discussion
The final typing scheme was established using 17 typing phages. Of the 617 S. Infantis strains analyzed, 605 could be typed with the established scheme, resulting in 61 different S. Infantis phage types (PT). The S. Infantis phage types PT 29 (30 %), PT 1 (21 %), PT 11 (7 %) and PT 9 (7 %), which are epidemiologically predominant, were found in humans and food as well as broilers.197 strains isolated from food poisoning (40) and hospital outbreaks (11) were subtyped into 12 defined phage types (Figure 1). Remarkable was the emergence of S. Infantis as a nosocomial pathogen in several German hospitals. The most frequently observed clone in these outbreaks was PT 29/XbaI 27. This clone was repeatedly found in a hospital in Baden-Württemberg since 2002. In 2004 a huge outbreak with 188 cases in two clinics was investigated in Bavaria and traced back to bakery products. By phage typing and macrorestriction analysis the isolates from humans and food have been characterized to be identical (clone PT 53/XbaI 6). An outbreak with 80 cases in North Rhine-Westphalia in 2008 was caused by contaminated chicken kebab (clone PT 1/XbaI 34). A supra regional outbreak of S. Infantis in Thuringia, North Rhine-Westphalia and Lower Saxony was confirmed through clone PT 29/XbaI 27a which was found in isolates from humans and broilers. Additionally, some diffuse outbreaks could be detected by this complex typing system.
Figure 1: Distribution of S. Infantis phage types in Germany reported from humans (A) and
broilers (B)
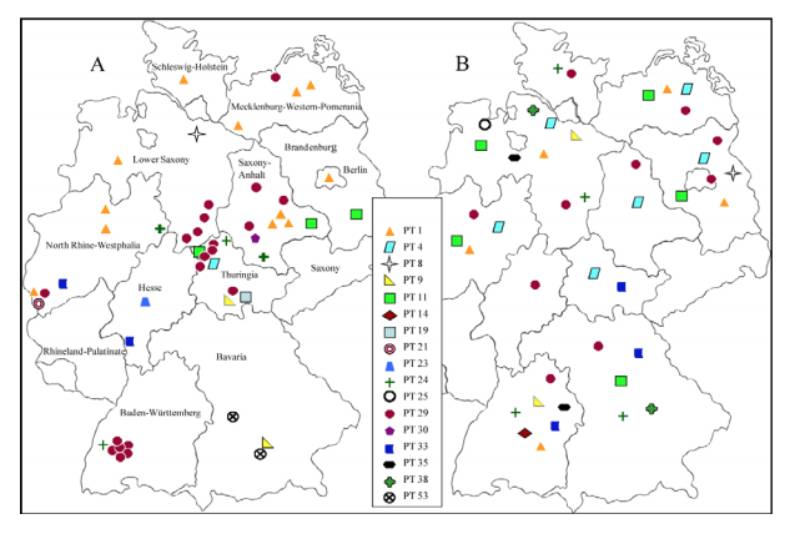
The EFSA studies have shown that the serovar S. Infantis is common in poultry. In this study broiler strains from Germany (n=143), Hungary (n=22), Australia (n=16), and Iceland (n=20) were subtyped using phage typing and macrorestriction analysis. A total of 24 phage types among these broiler strains were found.
In Germany clones PT 4/XbaI 4, PT 29/XbaI 5 and PT 29/XbaI 27 were frequently observed in isolates from broilers. The strains belonging to PT 29/XbaI 5 were associated with broilers imported from Hungary. This clone was found only in sporadic cases in Germany. Nogrady et al. (2007, 2008) reported recently that strains of PT 213 and PT 217 were most commonly found in Hungary, in humans as well as in broilers. The strains of PT 213 according to phage typing scheme of Laszlo correspond to PT 29 according to the phage typing scheme used at the NRC in Wernigerode. It is important to note that strains of PT 29/XbaI 27 from Germany and strains of PT 29/XbaI 5 from Hungary which are indistinguishable by phage typing differ in their macrorestriction patterns only in two bands of 70 kbp and 170 kbp, probably due to plasmids.
In contrast to the situation in Germany and Hungary, where clone PT 29 is epidemic among broilers, subtying of S. Infantis strains from broilers in Iceland showed a dominance of clone PT 61, which has not yet been observed in Germany. Pelkonen et al. (1994) were unable to confirm a relationship between isolates from humans and from poultry in Finland. S. Infantis repeatedly observed in cattle could explain epidemics in Finland during the 1970s (Lindqvist et al. 1999; Lindqvist & Pelkonen 2007). In the EFSA study it was also stated that in 2006 S. Infantis was the most frequent serovar found in beef (EFSA 2007c). By phage typing and macrorestriction analysis the spreading of specific S. Infantis clones in broiler flocks, the circulation of defined clones between countries, and the development of country-specific epidemiologic processes were detected.
In conclusion, the data presented in this study confirmed that S. Infantis strains are widespread in broiler flocks worldwide. In Hungary and Japan S. Infantis is the most frequent serovar in broilers (Nogrady et al. 2008; Ishihara et al. 2009; Noda et al. 2010). After successful vaccination against S. Enteritidis and S. Typhimurium in broiler flocks apparently other serovars may enter this ecological niche to persist whereas S. Infantis is a potential candidate. The phage typing scheme developed can be used for laboratory-based surveillance of S. Infantis.
Main References (complete list can be obtained from the senior author):
1. EFSA (2007a): Report of the Task Force on Zoonoses. Data Collection on the Analysis of the baseline study on the prevalence of Salmonella in holdings of laying hen flock. The EFSA Journal. 97:1-85.
2. EFSA (2007b): Report of the Task Force on Zoonoses. Data Collection on the Analysis of the baseline survey on the prevalence of Salmonella in broiler flocks Part A. The EFSA Journal. 98:1-85.
3. BfR (Bundesinstitut für Risikobewertung, Berlin) (2005): Pilotstudie zum Vorkommen von Salmonella spp. bei Herden von Legehennen in Deutschland.
4. BfR (2006): Grundlagenstudie zur Erhebung der Prävalenz von Salmonellen in Broilerbetrieben.
5. EFSA (2007c): The Community Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, Antimicrobial Resistance and Foodborne Outbreaks in the European Union in 2006. The EFSA Journal. 130:1-353.
6. EFSA (2009): The Community Summary Report on Trends and Sources of Zoonoses and Zoonotic Agents in the European Union in 2007. The EFSA Journal. 223:1-313.
7. Rabsch, W. et al. (2000): Competitive exclusion of Salmonella enteritidis by Salmonella gallinarum in poultry. Emerg. Infect. Dis. 6:443-448.
Zusammenfassung
Epidemiologische Zusammenhänge zwischen Salmonella Infantis-Isolaten von Mensch und MasthähnchenSalmonella (S.) Infantis-Infektionen des Menschen, oft durch Lebensmittel übertragen, sind weltweit von wachsender Bedeutung. S. Infantis-Stämme verschiedener Herkunft, die von 2002-2009 isoliert wurden, sind durch die Lysotypie und die XbaI- Makrorestriktionsanalyse (MRA) subtypisiert worden.
617 der mit Lysotypie untersuchten S. Infantis-Isolate gehörten zu 47 definierten Lysotypen, und die 255 S. Infantis-Isolate, die mit der MRA charakterisiert wurden, zu 45 XbaI-Typen. Die Kombination (komplexe Typisierung) der beiden Methoden beweist die erhebliche klonale Diversität der S. InfantisIsolate, zeigt aber auch das Vorkommen von bestimmten Klonen wie z. B. PT 1/XbaI 34 und PT 29/XbaI 27 sowohl bei Isolaten aus Masthähnchenfleisch als auch bei humanen Isolaten über Jahre hin in Deutschland. Es wurden auch mehrere Krankenhausinfektionen durch die benannten Klone in Deutschland erfasst. Die komplexe Typisierung wies weiterhin die Zirkulation des S. Infantil-Klons PT 29/XbaI 5 in Broiler-Beständen in Ungarn und Deutschland nach sowie die Existenz sog.
länderspezifischer epidemiologischer Prozesse wie z. B. in Island durch den Klon PT 61. Der offenbar durch die Vakzination gegen S. Typhimurium und S. Enteritidis begünstigte Erregerwechsel in der ökologischen Nische „Masthähnchen“ könnte in der Zukunft dazu führen, dass S. Infantis in Masthähnchenbeständen häufiger zum Problemkeim wird. Deshalb kann der routinemäßige Einsatz des neuen Lysotypieschemas für S. Infantis dazu beitragen, die zeitliche und geografische Ausbreitung dieses Erregers zu überwachen.
Acknowledgments
We thank B. Malorny and A. Schroeter from the Federal Institute for Risk Assessment in Germany, D. Davos from the Salmonella Reference Centre in Australia, N. Nógrády from the National Center for Epidemiology in Hungary and Thorunn R. Thorsteinsdottir from the Institute for Experimental Pathology, University of Iceland for the isolates from humans and poultry.
