Abstract
Strengthening the prudent use of antimicrobials by European veterinarians has resulted in an increasing interest in the use of autogenous vaccines. However until December 2018 autogenous vaccines were regulated on national level in the European member states resulting in different standards in the manufacture and quality control of autogenous vaccines. At the same time it is well known that vaccinated food-producing animals easily cross borders within Europe. Therefore not only for consumer protection reasons there is strong interest to regulate autogenous vaccines on a European level. In order to do so a new regulation on veterinary medicinal products was proposed by the European Commission and the Council of Europe in order to repeal the current Directive 2001/28/EC that excludes autogenous vaccines.
Keywords
Directive 2001/28/EC, veterinary medicinal products, autogenous vaccines, new regulation of the European Parliament and of the Council Regulation (EU) 2019/6 on veterinary medicinal products
Introduction
On 25/26th September 2017 a joint workshop titled “Autogenous Vaccines and Their Role in Animal Health Strategies”, organized by the International Alliance for Biological Standardization and the University of Gent, discussed the legal situation of autogenous vaccines in Europe in comparison to registered veterinary medicinal products. Representatives of the animal health industry and national authorities as well as the European Commission desired changes to the current legislation for veterinary medicinal products, based on experience with respect to the functioning of the veterinary medicines market, administrative burdens for registered products, scientific progress and protection of animal and public health.In contrast to registered vaccines, autogenous vaccines are always inactivated and only allowed to be used locally, on one holding under exceptional circumstances, when a registered vaccine against an indication in a target animal is not available. The market for autogenous vaccines therefore is rather small, but their fast availability allows the veterinarian to quickly respond to new diseases or to diseases, against which no registered product is available. Batches of autogenous vaccines are rather small as they are herd and customer specific products.
Legal Situation of AV in Europe
Commission Directive 2001/82/EC as amended regulates the manufacture, control, placing on the market, import, export, supply, use and pharmacovigilance of veterinary immunologicals. It guarantees the quality of veterinary medicinal products manufactured within the Community by requiring compliance with good manufacturing practice. Each veterinary medicinal product must be authorized before being placed on the market.Autogenous vaccines (AV) are also defined in this Directive 1), but are excluded from its scope. The consequence of this exclusion is that AVs are not regulated on a European level. Their quality, manufacture and use depend on the individual national legislation of each member state. Legislation for autogenous vaccines differs from member state to member state.
In Germany the Animal Vaccines Act of 2006 (TierImpfVO 2006) requires that manufacturers of AV have a manufacturing authorization, issued only after inspection by the local authorities responsible for GMP. All batches manufactured must be notified to the authority responsible for GMP and includes the identity of the prescribing veterinarian, the operation of destination, target species and quantity supplied. In addition the German federal states have published a so-called “Question and Answer Paper” which includes recommendations for manufacturers and users of veterinary autogenous vaccines. However, the recommendations are not legally binding. A vaccine registration procedure including dossier evaluation for AV is currently not required in Germany. Some EU-member states hardly have any equivalent regulations. Most extensively regulated are autogenous vaccines in the United Kingdom.
Legal Situation in the UK
AVs are regulated in the Veterinary Medicines Regulation 2013 as amended. Manufacturers and their product intended to be placed on the market must be authorized by providing to the Veterinary Medicines Directorate (VMD) an application dossier that includes detailed quality documentation. Further manufacturers must either provide a GMP-certificate or ask the VMD for an inspection of the production and quality control facilities as part of the approval procedure. The need and use of autogenous vaccines in preference to UK authorized products must be soundly justified by a UK veterinarian.Although mainly bacterial autogenous vaccines are used, also viral vaccines can be licensed. As the first manufacturer Vaxxinova GmbH in Cuxhaven received the license for the manufacture and use of autogenous Fowl-Adenovirus vaccine in the UK in 2015. Testing of viral isolates for extraneous agents as specified in the European Pharmacopoeia using validated methods is self-explanatory.
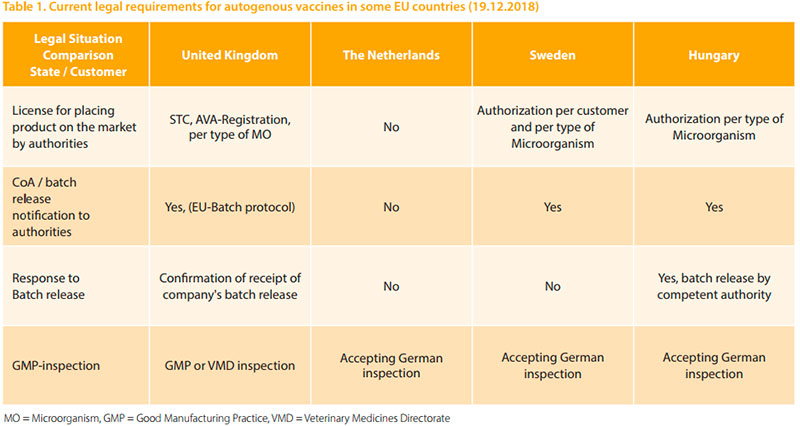
The mentioned UK-regulations on one hand seem to be a significant administrative burden to AV-manufacturers and veterinarians aiming to use AV in emergency situations. The consideration of the nature of the animal production industry on the other hand allows vaccine applications within larger epidemiological units and the use of the same isolate to produce further batches of vaccine on a quality risk basis. Each batch of vaccine must be tested for safety on-site on a small number of animals, before release of product. Adverse Reactions are notified to the Secretary of State within 15 days of learning the reaction. An overview of the requirements within some EU-countries is summarized in table 1.
Steps towards Harmonization in Europe
The “Recommendations for the manufacture, control and use of inactivated autogenous veterinary vaccines within the EEA” issued by the veterinary Coordination Group for Mutual recognition and Decentralised Procedures (20th March 2017) were a first step on European level to harmonize standards for autogenous vaccines. However these are currently not legally binding unless member states individually have included principles like these in their national law (e.g. the UK, Denmark, Hungary).In a second step the conclusions of the Gent workshop were directly forwarded to the European Commission and included in a compromise “Proposal for a Regulation on veterinary medicinal products” by the European Parliament and the Council of the European Union on 5 June 2018. The new Regulation came into force in Jan 2019.
Conclusion of the Workshop and the new REGULATION 2019/6 on veterinary medical products OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Regulation (EU) 2019/6 on veterinary medicinal products repealing Directive 2001/82/EC
The joint workshop in Gent agreed that AVs are useful and necessary.AVs should be defined as: inactivated immunological veterinary medicinal products which are manufactured from pathogens and antigens obtained from an animal or animals in an epidemiological unit and used for the treatment of that animal or those animals in the same epidemiological unit or for the treatment of an animal or animals in a unit having a confirmed epidemiological link. (Chapter I Subject matter, scope and definitions, Article 2 Scope (3)).
The quality of autogenous vaccines should be harmonized. Although autogenous vaccines should be manufactured in accordance with the principles of good manufacturing practice, detailed guidelines of good manufacturing practice should specifically be prepared for those products since they are manufactured in a way that is different from industrially prepared products. That would preserve their quality without hindering their manufacturing and availability.
These special GMP-guidelines for autogenous vaccines however still need to be prepared: The obligations regarding certificates of good manufacturing practice for AVs shall only start to apply from the date of application of the implementing acts laying down specific measures on good manufacturing practice for those products. (Chapter XII Transitional and final provisions, Article 159 Transitional provisions regarding certain certificates of good manufacturing practice)
AVs shall only be used in the animals referred to therein in exceptional circumstances, in accordance with a veterinary prescription, and if no immunological veterinary medicinal product is authorised for the target animal species and the indication. (Chapter VII Supply and use, SECTION 3 USE, Article 106 Use of medicinal products (5)).
The advertising of autogenous vaccines shall be prohibited.(Chapter VII Supply and use, SECTION 4 ADVERTISING, Article 120 Advertising of veterinary medicinal products subject to veterinary prescription (3).
Also for autogenous vaccines Member States shall ensure that appropriate systems are in place for the collection and disposal of waste of veterinary medicinal products.( Article 117 Collection and disposal of waste of veterinary medicinal products)
Animal keepers and owners of foodproducing animals must keep record of the use of autogenous vaccines (Article 108 Record-keeping by owners and keepers of food-producing animals)
Competent authorities shall carry out controls of manufacturers, distributors, marketing authorisation holders, wholesale distributers, retailers, owners and keepers of food-producing animals, veterinarians, registration holders and any other persons having obligations under this Regulation. (Chapter VIII Inspections and controls, Article 123 Controls)
The new regulation facilitates the use of registered vaccines.
Licensing of vaccines shall be facilitated e.g. by ensuring mutual recognition of national authorizations by use of special procedures.Administrative burdens shall be reduced by full in-depth assessment of an application only once.
Veterinarians should be allowed by way of exception to prescribe other medicinal products, if authorized medicinal products are not available.
For limited markets and under exceptional circumstances it should be possible to grant marketing authorizations without a complete application dossier, based on a benefit-risk assessment of the situation, e.g. for minor species or for diseases that occur infrequently or in limited geographical areas. The validity of these authorizations shall be 5 years for limited markets and 1 year for exceptional circumstances.
Summary and Conclusions
The new regulation 2019/6 by European Commission and Council of Europe repeals Commission Directive 2001/82/EC and will result in harmonized standards of manufacture and quality control of autogenous vaccines within Europe, which are still to be defined however. Autogenous vaccines are manufactured in a way that is different from industrially prepared products. It is emphasized that the new standard will preserve their quality without hindering their manufacturing and availability. Another important outcome of the new regulation is, that also for new veterinary medicinal products under GMP the way to market via registration will be facilitated. Altogether this should result in a better availability of vaccines to veterinarians in Europe.
References
Commission Directive 2001/82/ECRegulation (EU) 2019/6 on veterinary medicinal products https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0006&from=EN
Recommendations for the manufacture, control and use of inactivated autogenous veterinary vaccines within the EEA (2017):CMDv, London, 20 March 2017, EMA/CMDv/452656/2016, REC-002-01
Frage-Antwort-Papier zur Überwachung der Herstellung von bestandsspezifischen Impfstoffen (2018): Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten, 09.06.2018, www.zlg.de https://www.zlg.de/index.php?eID=dumpFile&t=f&f=1479&token=7cef939c7598467ac2e1f9f8bf368ae86d93b0c3
