Abstract
During the last decades meat and especially liver have been looked upon as unhealthy food with high fat content and cancerogenic potential. In addition, its content of highly valuable micronutrients has mostly been ignored. As a result, the mean uptake and serum levels of several micronutrients in the population are below the recommended levels. In the meantime, the contamination of liver with heavy metals and other contaminants has fallen far below the allowed thresholds and sometimes even below the detection limit while its content of micronutrients like iron, folate, selenium or zinc are still high. As a further advantage, the bioavailability of many micronutrients often is better from meat and liver than from plant sources. These advantages and the low content of contaminants in meat and liver leads us to conclude that meat and liver should be considered as a regular part of mixed diets with vegetables and fruits as the major components to ensure an optimal supply with micronutrients.
Keywords
Micronutrients, meat, liver, human nutrition, human health
Introduction
Many people consider meat, especially red meat, as unhealthy food, mainly due to its postulated “high” fat content and its very recently posted “reputation” that red meat is a promoter of colon cancer. As a consequence, low or no meat intake is often recommended to avoid or minimize the risk of cancer, obesity and metabolic syndrome. Ethical arguments against intensive animal farming and its impact on global climate change contribute to the negative image of meat, but will not be addressed in this review.These more or less justified arguments against meat consumption ignore the fact that meat is a valuable source for some essential micronutrients such as iron, selenium, vitamins A, B12 and folic acid. Some of these are not present in plants at all or have such a low bioavailability that eating only plant-derived food may be inadequate to meet the daily requirement.
The fact that the world wide inadequacy of some micronutrients (hidden hunger) is related primarily to iron, zinc, vitamin A and folic acid is a result of rather poor diet diversity with the major component being starchy food and with a very low supply of meat and meat derived products (Biesalski 2013). Meat is an important food as an essential part of a mixed diet that ensures adequate delivery of micronutrients and amino acids even in low amounts. If meat and meat derived products are completely avoided the risk of inadequate supply and subsequent health relevant negative effects (increased infectious diseases, impairment of the immune system, anemia etc.) may occur.
Meat as an important source of micronutrients
Vitamin A and vitamin B12 occur only in meat and can hardly be compensated for by plant-derived provitamins: a provitamin B12 does not exist and provitamin A, ß-carotene, would have to be taken up in highest amounts to meet the daily vitamin A recommendation (1mg) due to its poor conversion rate of 1:12 (12 mg provitamin A needed to achieve 1 mg Vitamin A).Iron has a higher bioavailability when derived from meat as hem iron than plant-derived non-hem iron. Similarly, folic acid from meat (especially liver) and eggs has a nearly ten-fold higher bioavailability than from vegetables. Consequently low or no intake of meat (including liver) poses a risk for inadequate supply and symptoms of deficiency. Meat is also an excellent source for Thiamin (B1) and Riboflavin (B2). These nutrition facts lead to the question whether people with “normal” eating habits or only special groups of people are at risk for deficiencies of meat-derived micronutrients.
One risk group are elderly people who are generally considered at risk to develop vitamin and trace element deficiencies, especially for the vitamins A, D, E, and for folate as well as iron and calcium (Anderson 2001; Bates et al. 2002; Martins et al. 2002; Viteri and Gonzalez 2002). Recently a meta-analysis summarized the data from 41 studies with community-dwelling older adults (ter Borg et al. 2015). 30 % were below the Estimated Average Requirement (EAR) for Vitamin A, 95 % for vitamin D, 40 % for folate, Vitamin B1 and B2 and 15 % for iron. Per definition 50 % below the EAR and 50 % above the EAR are not at risk for deficiency. Adding two standard deviations to the EAR is used to calculate the reference value. Consequently, according to the definition of the EAR a supply below results in an increasing risk for developing a deficiency with clinical consequences. Malnutrition is far more common among institutionalized and chronic hospitalized elderly compared to free-living subjects in the community and the prevalence of malnutrition is associated with the severity of morbidity, functional impairments and mental state. Thiamine and folate status need special attention in this respect, as a deficiency of these nutrients is associated with depression and impaired cognition and dementia. Indeed, supplementation with B-vitamins resulted in a significant positive impact on mood and cognitive vigilance (von Arnim et al. 2013).
Pregnant women are another group at risk for micronutrient deficiencies. Their adequate nutrition plays an important role in the well-being of mother and child and also influences the health of the offspring not only during his intrauterine life but also during childhood and adolescence (intra- uterine or prenatal programming). While the enhanced requirements during pregnancy are met by a balanced diet and physiological adaptive processes, the micronutrient status of vitamin A, D, folic acid, iron, and zinc may become compromised without supplementation (Draper et al. 1993; Fogelholm 1999; Saletti et al. 2000) especially when meat is avoided, which can be frequently seen in women in the child bearing age.
Selected micronutrients from meat and liver
Meat and liver are excellent sources for a number of micronutrients: low-fat pork contains 1.8 mg iron and 2.6 mg zinc; pig liver contains 360 mg magnesium, 20 mg iron and 60 μg selenium per 100 g. Thus, meat and liver (100g/day) can cover up to 50 % of the Recommended Daily Allowance (RDA)1 for iron, zinc, selenium, vitamins B12, B1, B2, B6, and 100 % of vitamin A. Another reason to include liver in the diet is that newest results show porcine as well as bovine liver to be almost free of harmful substances like heavy metals (a former reason to avoid the uptake of liver) which are far below the allowed threshold or even below the technical detection limit (DGE 2004).Vitamin A
The best source for vitamin A is liver. Liver of pork and beef contains 6.0 μg and 20 μg of vitamin A respectively (table 1). However, liver consumption is low and restricted to some areas in Germany where it is a traditional food. The argument that liver should be avoided in a healthy diet has been based primarily on suspected contaminants in the liver (e.g. hormones, xenobiotics, metals etc.) which nowadays are seldom detected (see above). If ß-carotene from vegetables were the only source of vitamin A, more than 500 g mixed and ß-carotene rich vegetables per day must be consumed to reach the recommended 1 mg retinol. This amount of vegetables may actually contain more contaminants than a portion of liver, as their concentration in vegetables and fruits seem to have increased during the late 1990th and early 2000th (DGE 2004). A small portion of liver (100 g) twice a month supplies sufficient vitamin A and is neither toxic nor teratogenic. A poor vitamin A-status which cannot be easily detected via blood analysis due to its homoeostatic regulation increases the risk for respiratory tract diseases and impairment of the immune system (Biesalski 2013).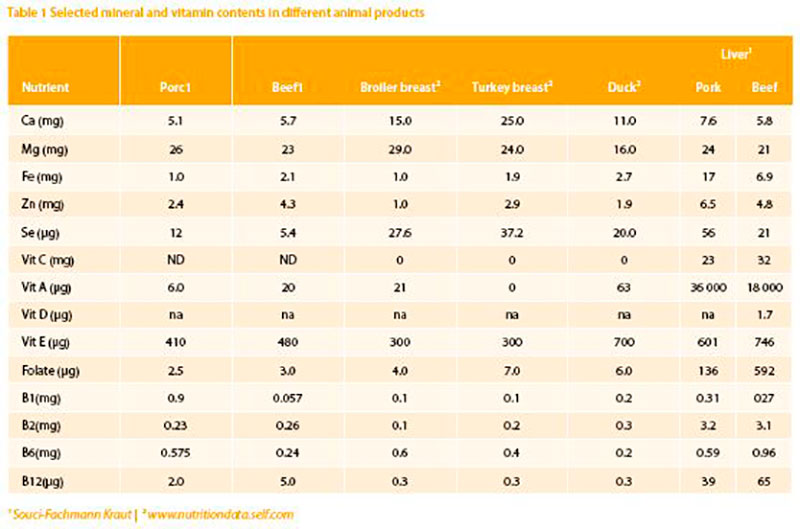
Folate
In European countries the average folate intake in adults was found to be remarkably similar, around 300 μg/day in adult males and 250 μg/day in adult women (De Bree et al. 1997). This is below the former recommended intake level of 400 μg/day (which has recently been reduced to 300 μg/day) and too low for pregnant women and women planning a pregnancy. For these groups an intake of 600 μg/ day is considered protective against Neural Tube Defects (NTD), which appears in around 700 cases/year in Germany. More than 90 % of women of child bearing age have sub-optimal dietary folate intake. Supplementation of folate to the optimal level can reduce the risk to develop NTD by up to 70 %. Data regarding meat derived folate intake and NTD is not available. But it should be considered that meat and liver in particular are sources with better bioavailability than plant derived folate. Due to the fact that females in child bearing age are not adequately supplied with folate, supplements (400 μg/day) are strongly recommended.Vitamin B12
Vitamin B12 is found only in animal products. In a recent UK study of 250 vegetarian and 250 vegan men, approximately one quarter of vegetarians and more than half of vegans had sub-optimal intakes of vitamin B12. Plasma vitamin B12 levels were low in the vegetarians and extremely low in the vegan group, with more than a quarter below the threshold level where neurological signs may develop (Dror and Allen 2008; Gilsing et al. 2010). Elderly nonvegetarian people are also at risk of vitamin B12 deficiency, due to physiological changes resulting in reduced absorption. In the UK, vitamin B12 status in some people aged 65 and over was inadequate. There was no difference in mean levels between men and women. However, vitamin B12 intake was adequate when compared with UK dietary reference values (Finch et al. 1998). To assure the RDA of vitamin B12 (3 μg/day for adults), again meat (bovine 5 μg/100 g) and especially liver (depending on the species 30-65 μg/100 g) are the best sources.Iron
Although iron is one of the most abundant elements in the Earth‘s crust, iron deficiency is the most common and widespread nutritional disorder in the world. According to the WHO (2008) Iron deficiency adversely affects- the cognitive performance, behaviour, and physical growth of infants, preschool and school-aged children;
- the immune status and morbidity from infections of all age groups;
- the use of energy sources by muscles and thus the physical capacity and work performance of adolescents and adults of all age groups.
- specifically, iron deficiency anemia during pregnancy increases perinatal risks for mothers and neonates; and increases overall infant mortality.
Moreover, iron-deficient animals and humans have impaired gastrointestinal functions and altered patterns of hormone production and metabolism. The latter include those for neurotransmitters and thyroidal hormones which are associated with neurological, muscular, and temperature- regulatory alterations that limit the capacity to maintain their body temperature of individuals exposed to cold.
The iron-concentration in meat is between 1-3 mg/100g. Liver contains between 8 mg/100 g (calf ) and 16 mg/100 g (pig). In bread and legumes, the concentration varies between 1 mg up to 8 mg/100 g. Due to biological losses, such as cyclical monthly bleeding of fertile-aged women, excessive infestation with blood-feeding parasites, or poor bioavailability of iron from plant-based diets, it is estimated that as many as 3-4 billion people, around 20 % in high income and 50-60 % in low income countries may have inadequate iron supply with the diet. At any given time, 1.62 billion people – over 30 % of the world’s population – are anemic, mainly due to iron deficiency. In developing countries this is frequently exacerbated by malaria and worm infections (WHO, 2008). The highest prevalence (47.4%) has been found in preschool children and nonpregnant females.
In a recent Irish food consumption survey, almost half of women aged 18-50 year had inadequate iron intakes when compared with national average requirements. Around 25 % of all pregnant women in Europe suffer from iron deficiency. The daily supply is below 60 % of the estimated average requirement (EAR) for pregnancy (Blumfield et al. 2013). Breastmilk is low in iron, in particular when the supply of the mother is inadequate. In a recent study in Germany 21 % of breast fed children were iron deficient and 6 % had clear symptoms of anaemia (Dube et al. 2010). During postweaning age (12 – 23 months) 15 % of male and 18.5 % of female children in Germany had an inadequate supply (below EAR) (Hilger et al. 2015). In the British National Diet and Nutrition Survey, iron intake was found to be low in girls (aged 7-18 years) and decreased with age. Adolescent females (15-18 year) were found to have extremely low intakes of iron when compared to UK dietary reference values.
Depending on the composition of the individual diet the bioavailability of iron can differ 5 to 10-fold. The bioavailability depends on the presence or absence of different ligands (phytates from cereal products, tannins from coffee and tea and oxalates from vegetables) which form complexes with iron and zinc and block their absorption. Diets primarily composed of vegetables, rice, beans and corn are associated with poor iron bioavailability. This explains the high incidence of anaemia in developing countries. 100 g pork meat and an even lower amount of liver added to a vegetarian diet increases the iron absorption 3.6 fold.
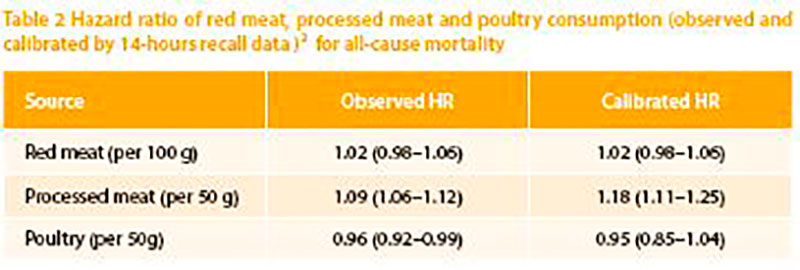
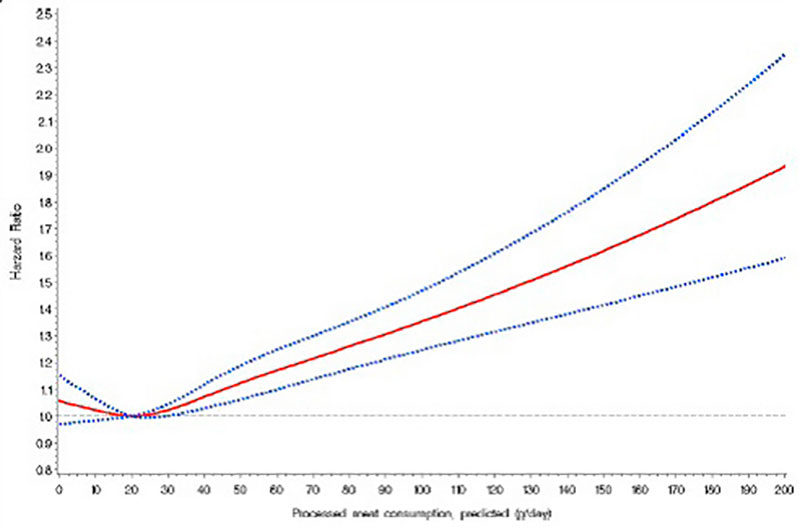
Figure 1: Nonparametric regression curve for the relation of processed meat intake at recruitment with all-cause mortality, European Prospective Investigation into Cancer and Nutrition (EPIC) 1992-2009. Solid line: effect estimate; dotted lines: 95 % confidence interval (Rohrmann et al. 2013). (We acknowledge the permission to reprint the graph)
Zinc
Zinc is essential for more than 100 enzymes and plays an important role within the immune system. Zinc deficient individuals demonstrate slower wound healing and are more prone to infections. Some studies showed that zinc may beneficially affect cold symptoms; however a metaanalysis of randomized controlled trials concluded that there is no evidence for the effectiveness of supplemented zinc in reducing the duration of common cold symptoms (Jackson et al. 2000). Especially in elderly a reduced zinc status is evident (Lukito et al. 2004). In the same group, a higher protein intake (together with slight exercise) stopped sarcopenia, a progressive loss of lean body mass. The RDA for zinc is about 12-15 mg/day. A higher need of zinc is documented (20-25 mg/day) during pregnancy and lactation as well as during chronic inflammatory diseases (Rink and Gabriel, 2000).Meat contains between 2 and 10 mg of zinc/100g. Plant derived food is also a good source, in particular green leafy vegetables (up to 70 mg/1000 kcal) but due to the more reliable bioavailability meat seems a safer source of zinc.
Considering the nutrition value of vegetarian diets, beside vitamin B12, iron and zinc are the micronutrients of greatest concern. Bioavailability of iron and zinc is substantially decreased through the interaction with phytate and further constituents of plant food.
Meat and cancer
Meat consumption, especially red meat, is not carcinogenic per se, even if it contains components which are assumed to contribute to cancer formation. On the other hand, persons with a high intake of fruit and vegetables are considered to have a reduced cancer risk because of protecting factors such as carotenoids, flavonoids, further phytochemicals and also folic acid, selenium, zinc and other components. It is not clear why these latter compounds should be less effective if they reach the body via meat. The balance of promoting and protecting factors within the diet is important for the protection against cancer. Furthermore, the insulin-resistancehypothesis shows that a nutritional behavior leading to a metabolic syndrome (high energy, high glycaemic carbohydrates) might favor colon cancer or even other types of cancer. The “goals for nutrition in the year 2000” (Willett, 1999) give a very good and comprehensive advice: “Current nutritional recommendations for the prevention of cancer include increased consumption of fruits and vegetables; reduced consumption of red meat and animal fat; and avoidance of excessive alcohol”.Very recently the WHO classified red meat as class 2A carcinogenic based on epidemiological data. Class 2A means that there is sufficient evidence of carcinogenicity in humans. However, the data used are based on limited evidence from epidemiological studies. Limited evidence describes a result which shows an association between exposure of red meat and cancer but other reasons for the increased risk cannot be excluded. This limited evidence is a conclusion in all meta-analysis dealing with red meat intake and cancer risk (Alexander et al 2015; Egeberg et al 2013; Lippi et al 2015; Pham et al 2014). Different epidemiological studies between 2005 and 2013 were used to justify the relation between high meat intake and increased risk for Colorectal Cancer (CRC) as the basis for the cancer risk classification. The European Prospective Investigation into Cancer and Nutrition (EPIC) study, a big human cohort study with more than 500.000 participants showed that the highest daily intake of meat (160 g/day) has a relative risk of 1.35 compared to the lowest intake (Norat et al. 2005). Red meat alone (> 80 g/day) increased the risk slightly (1.17 %), processed meat (>80 g/day) increased the risk up to 1.42 %. A meta-analysis (Chan et al. 2011) with 10 cohort studies documented a relative risk for the highest intake (140 g/day) of 1.14 % compared to the lowest. Further increase of meat consumption did not change the relative risk. Based on that study the International Agency for Research on Cancer (IARC) distributed the warning and the risk classification (Bouvard et al. 2015). A more detailed examination of the EPIC cohort, however, showed a more differentiated result:
Depending on the model used (uncalibrated or calibrated), only processed meat showed a risk increase of 50 g additional meat/day. The impact of daily processed meat consumption on the risk to develop cancer shows a Ushaped pattern. The lowest consumption of processed meat has the same mortality as a daily intake of approx. 55 g.
In contrast, red meat consumption in the highest quartile vs. lowest did not show a significant increase in cancer mortality. There was no association between intake of poultry and all-cause mortality.
A very recent meta-analysis with 27 prospective studies and a broader evaluation of the relative risk estimates by specific intake levels documented again a rather weak relation between red meat consumption and CRC risk (Figure 2).
At intake levels > 100 g red meat per day showed a slightly increased risk (1.20 %). The elevation of the relative risk related to servings per day is largely influenced by male participants. Alexander et al. (2015) concluded:
In the current meta-analysis of red meat intake and CRC (colorectal cancer), we comprehensively examined associations by creating numerous subgroup stratifications, conducting extensive sensitivity analyses, and evaluating dose-response using several different methods. Associations between red meat and CRC were weak to nonexistent. Of the highest categories of intake in the individual studies, over onethird of the RRs (relative risks) were 1.0 or lower, and almost half were less than 1.05. Summary associations were weak, with most SRREs (summary relative risk estimates) around the null value or just slightly above. Because of multiple comparisons (i.e., over 70 separate analyses were conducted), some statistically significant correlations could arise by chance alone.
In conclusion, based on the quantitative findings and scientific rationale for interpretation documented in the current metaanalysis, red meat intake does not appear to be an independent predictor of CRC risk“.
The relation between slight but insignificant risk increase and servings per day shows that the best way to be on the save side with respect to either cancer risk or inadequate micronutrient intake is a mixed and balanced diet. Indeed, a simple and very old suggestion.
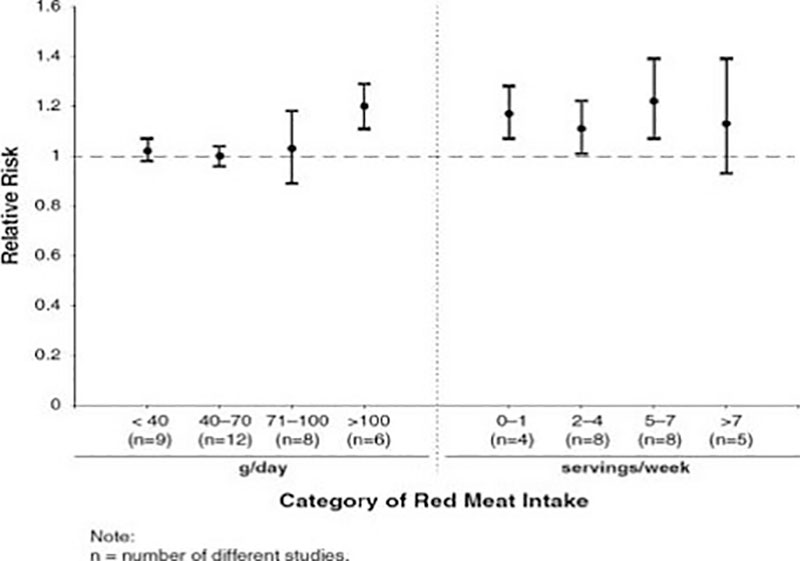
Figure 2: Dose response patterns showing the relativ risk to develop Colorectal Cancer (CRC) based on meta-analyses of categorical intake groupings (adapted from Alexander et al. 2015)
Conclusions
Any kind of an unbalanced diet, either avoiding meat or fruit or vegetables may lead to (micro-) nutrient deficiencies and consecutive disorders especially in groups with higher needs (pregnant or lactating women, excessive exercise, growing children, elderly, etc.). At this time one has to assume, that a mixed and balanced diet with higher amounts of fruits and vegetables and moderate amounts of meat (processed and unprocessed) including liver, accompanied with physical exercise is recommended and seems to be the best recipe for prevention of nutritional deficiencies and physiological malfunctions.

References
Anderson AS (2001). Pregnancy as a time for dietary change? The Proceedings of the Symposium on ‘nutritional adaptation to pregnancy and lactation’Nutrition Society, 60:497-504.Alexander DD et al (2015) Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr 34:521-543
Armin C von et al (2013) Micronutrients supplementation and nutritional status in cognitively impaired elderly persons: a two-month open label pilot study Nutrition Journal 12:148
Bates CJ, Benton D, Biesalski HK, Staehelin,HB, van Staveren W, Stehle P, Suter PM & Wolfram G (2002) Nutrition and aging: a consensus statement. The Journal of Nutrition, Health & Aging, 6:103-116.
Biesalski HK (2013) Hidden Hunger. Springer (Heidelberg)
Blumfield ML et al (2013) Micronutrient intakes during pregnancy in developed countries: systematic review and meta analysis. Nutr Rev 72:118-132
Bouvard V et al (2015) Carcinogenicity of consumption of red and processed meat. The Lancet Oncology 16:1599-1600 Chan D et al (2011) Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLo- SOne e20456
De Bree A, van Dusseldorp M, Brouwer IA, van het Hof KH & Stegers-Theunissen RPM (1997). Folate intake in Europe: recommended, actual and desired intake. The European Journal of Clinical Nutrition, 51:643-660.
DGE, Deutsche Gesellschaft für Ernährung (2004) Ernährungsbericht 2004.
Draper A, Lewis J, Malhotra N & Wheeler E (1993) The energy and nutrient intakes of different types of vegetarian: a case for supplements? The British Journal of Nutrition, 69:3-19.
Dube K et al (2010) Iron intake and iron status in breastfed infants during the first year of life. Clin Nutr.29:773-778
Dror DK, Allen LH (2008) Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 66: 250–255
Egeberg R et al (2013) Associations between red meat and risks for colon and rectal cancer depend on the type of red meat consumed Journal of Nutrition 143:464-472
Finch S, Doyle W, Lowe S, et al. (1998) National Diet and Nutrition Survey: People Aged 65 Years and Over. Volume 1: Report of the Diet and Nutrition survey London. The stationary office
Fogelholm M (1999) Micronutrients: interaction between physical activity, intakes and requirements. Public Health Nutrition, 2:349-356.
Gilsing, AMJ, Crowe FL, Lloyd-Wright Z et al. (2010) Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr 64: 933–939 Hilger J et al (2015) Miconutrient intake in healthy toddlers: a multinational perspective. Nutrients 7:6938-6955
Jackson JL, Lesho E & Peterson C (2000) Zinc and the common cold: a meta-analysis revisited. The Journal of Nutrition, 130:1512-1515.
Lippi G et al (2015) Red meat consumption and ischemic heart disease. A systematic literature review. Meat science 108:32-36
Lukito W, Wattanapenpaiboon, N., Savige, G. S., Hutchinson, P. & Wahlqvist, M. L. (2004). Nutritional indicators, peripheral blood lymphocyte subsets and survival in an institutionalised elderly population. Asia Pacific Journal of Clinical Nutrition, 13(1), 107-112.
Martins I, Dantas A, Guiomar S & Amorin JA (2002). Vitamin and mineral intakes in the elderly. The Journal of Nutrition, Health & Aging, 6:63-65.
Norat T et al (2005) Meat, fish and colorectal cancer risk: the European prospective investigation into cancer and nutrition. J Natl Cancer Inst 97:906-916
Pham NM et al (2014) Meat consumption and colorectal cancer risk: An evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 44:651-650
Rink L and Gabriel P (2000). Zinc and immune system. The Proceedings of the Nutrition Society, 59(4): 541-52.
Rohrmann et al (2013) Meat consumption and mortality – results from the European Prospective Investigation in Cancer and Nutrition. BMC Medicine 11:63
Saletti A, Lindgren EY, Johansson L & Cederholm T (2000). Nutritional status according to mini nutritional assessment in an institutionalized elderly population in Sweden. Gerontology, 46:139-145.
ter Borg S et al (2015) Micronutrient intakes and potential inadequacies of community- dwelling older adults: a systematic review. BJN 113: 1195-1206
Viteri FE & Gonzalez H (2002) Adverse outcomes of poor micronutrient status in childhood and adolescence. Nutr Rev. 60(5 Pt 2):77-83
Willett WC (1999) Goals for nutrition in the year 2000. CA Cancer J Clin 49:331-352.
WHO (2008) Worldwide prevalence of anemia 1993-2005. WHO Library
