Abstract
Consumers and food business operators are more and more aware of food safety issues. Food safety can broadly be divided into microbiological and chemical food safety. Microbiological contamination of eggs has important implications. For table eggs, internal contamination may occur, leading to spoilage and in the case of a pathogen to human disease and food safety risks. Eggs are one of the main sources of contamination cited in relation to human salmonellosis, with Salmonella Enteritidis (SE) being the most frequently isolated Salmonella serovar. There are two possible routes of bacterial infection of table eggs: either vertically or horizontally. In the vertical transmission the egg content is directly contaminated as a result of bacterial infection of the reproductive organs, i.e. ovaries or oviduct tissue (transovarian route). In the horizontal transmission the micro-organisms penetrate through the eggshell. SE seems to have special capabilities to colonize the hen’s reproductive tract and to survive in the egg albumen compared to other Salmonella serovars and other bacteria. Several studies support also the idea that the vertical contamination route may be more important for SE. The causative agent of strong-evidence human outbreaks caused by eggs and egg products in the EU in 2012 is SE, with 66.7% of the cases.
Different factors play a role in the risks for spoilage and Salmonella contamination of eggs. Some of those factors will be discussed, starting with different physical and chemical defence mechanisms that protect egg contents from microbial invasion and multiplication. Egg factors (eggshell and egg content), egg freshness, egg cooling, eggshell condensation, amount of bacterial load on the shell, bacterial species, housing system, extending the self-live of eggs, … are important factors. Finally risk factors for SE contamination of laying hen farms and the impact of the Salmonella control programs in the EU, with or without compulsory vaccination against SE, will be discussed.
Keywords
Table eggs, microbiology, spoilage, Salmonella, risks
Introduction
The egg has several physical and chemical defence mechanisms that protect its contents from microbial invasion and multiplication. While the eggshell and shell membranes physically hinder microbial penetration into the egg albumen, the vitelline membrane reduces penetration into the most nutritious compartment of the egg, the egg yolk. The chalazae also act as physical barriers against bacterial penetration into the egg yolk maintaining the yolk in a central position. Finally, the various antimicrobial properties of egg albumen, its viscosity and alkaline pH inhibit bacterial proliferation and contribute to hindering egg yolk invasion. The properties of the main antimicrobial proteins present in the albumen are listed in Table 1.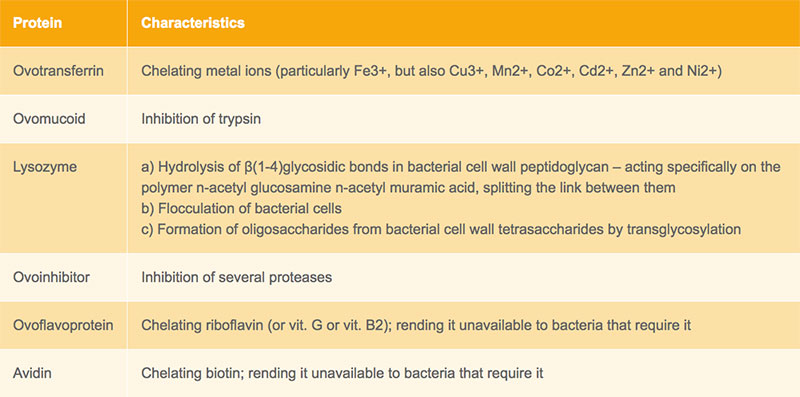
Table 1: Properties of the main antimicrobial proteins present in the albumen (De Reu 2006a)
Material and methods
To study the bacterial eggshell penetration and egg content contamination and the risks factors for eggshell penetration and egg content contamination different lab scale methods can be used and found in literature. Methods which, however, are often applied are following:1) An agar method that can be used to study and visualize the bacterial eggshell penetration and
2) An intact egg method to study the egg content contamination. Both methods are described by De Reu et al. (2006b and 2006c). Bacterial inoculation of the eggshell can be done by immersion of the egg (agar filled or intact) in solutions containing a certain concentration (e.g. 10^5 – 10^6 CFU/ml) of the bacterial target species. More details of this inoculation method is described by De Reu et al. (2006b and 2006c). Inoculation can also be done using a cotton swab. After immersion of the swab in an overnight bacterial culture, the contaminated swab can be used to inoculate the eggshell surface area. In short, the agar method (figure 1) consists of replacing the egg content by sterile molten Nutrient Agar, containing streptomycin, cycloheximide (preventing yeast and mold growth) and the indicator 2,3,5- triphenyl-tetrazolium-chloride. The addition of streptomycin to the agar assures that only the on the shell inoculated streptomycin resistant bacterial species are able to grow on the agar. Where bacterial eggshell penetration occurs, the target bacterium grows on the agar and reduces the TTC to the red colored formazan (figure 1).

Figure 1: Agar method – Filling up drained eggs with supplemented Nutrient Agar (left); inoculation of eggshell by immersion in a bacterial suspension (middle); visualisation of penetration by candling (right). De Reu et al. (2006b and 2006c)
Results and discussion
Risks for spoilage and Salmonella contamination of table eggs
Contamination routesThere are two possible routes of bacterial infection of shell eggs: either vertically or horizontally. In the transovarian route (vertical transmission, figure 2), the yolk (very infrequently the yolk itself), the albumen and/or the membranes are directly contaminated as a result of bacterial infection of the reproductive organs, i.e. ovaries or oviduct tissue, before the egg is covered by the shell. Vertical transmission can originate from infection of the ovaries of a laying hen via systemic infection, or from an ascending infection from the contaminated cloaca to the vagina and lower regions of the oviduct. Different researchers are convinced that the vertical route is the most important route for Salmonella Enteritidis (SE) contamination of eggs. This is due to the ability of SE to colonize the ovary and oviduct of laying hens long-term. (De Reu et al. 2008)
In the horizontal transmission the micro-organisms penetrate through the eggshell. The egg passes through the highly contaminated cloaca area at the moment of lay; this is often illustrated by visible faecal contamination on the shell. Following oviposition, the shell acquires contamination from all surfaces with which it makes contact. While being wet and entering an environment with a temperature of approximately 20°C below the hen’s body temperature, the egg will cool immediately. The egg content will contract and a negative pressure establishes inside the egg, thereby moving bacteria through the shell. However, the egg presents a complex series of defensive barriers to the contaminating organisms and although microbes may successfully penetrate the shell of the egg, further development may be arrested or delayed (De Reu et al. 2008).
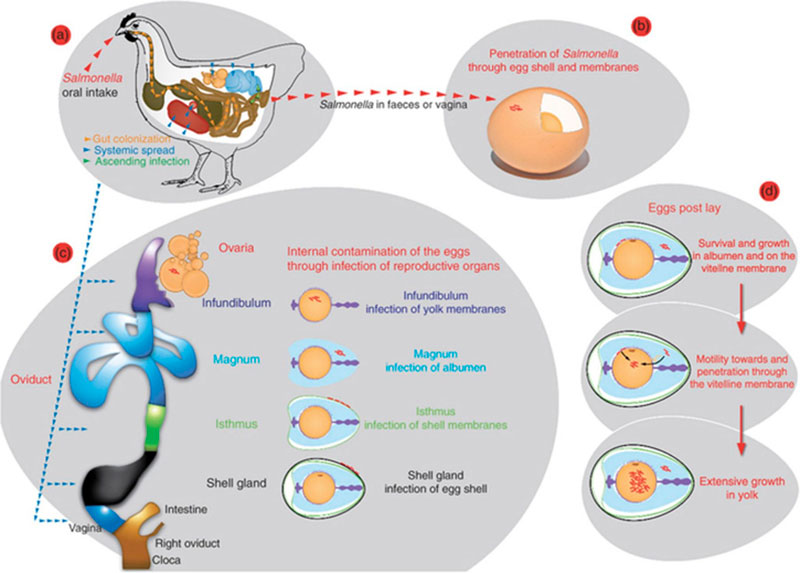
Figure 2: Overview of egg contamination by Salmonella (Gantois et al., 2009): a) Salmonella contamination of the reproductive organs of a hen via systemic spread after gut colonization or via an ascending infection; b) horizontal transmission route; c) vertical transmission route; d) survival and growth of Salmonella in the egg contents.
Type of bacterial flora
The microflora of the eggshell is dominated by Gram-positive bacteria, whereas Gram-negative bacteria are best equipped to overcome the antimicrobial defences of the egg content. Because of their tolerance for dry conditions Gram-positive bacteria which may originate from dust, soil or faeces can survive on the shell. Rotten eggs normally contain a mixed infection of Gram-negative and a few Gram-positive organisms. Some of the most common spoilage types are members of the genera Alcaligenes, Pseudomonas, Escherichia, Proteus and Aeromonas (Mayes and Takeballi, 1983; Board and Tranter, 1995). This indicates that Gram-negative bacteria are well equipped to overcome the antimicrobial defences of the egg. According to Board and Tranter (1995), the internal properties of eggs favour survival and growth of contaminating organisms which are Gram-negative, have a relatively simple nutritional requirement and have the ability to develop at low temperatures.
De Reu et al. (2006d; 2006e; 2007) have found that the natural eggshell contamination of table eggs was dominated by Gram-positive Staphylococcus spp.. Staphyloccoccus also seems to be the most dominating microflora in the air of the poultry houses (De Reu et al., 2007). As major egg content contaminants Gram-negative bacteria as Escherichia coli, Salmonella and Alcaligenes sp. were found. Beside also Gram-positive bacteria like Staphylococcus lentus, Staphylococcus xylosus and Bacillus sp. were isolated from the egg content (De Reu et al., 2006e, 2007, 2008).
Influence of bacterial load on eggshell penetration and egg content contamination
De Reu et al. (2006c) found that the probability of eggshell penetration is correlated with the eggshell contamination; but less obviously with the egg content contamination (figure 3). External eggshell contamination seems to be important for the shelf life and the food safety of consumption eggs and egg products. It is hypothesised that an important part of the bacterial contamination of the egg content results from the penetration of the shell by bacteria deposited on the surface of the egg after it has been laid (horizontal infection route).
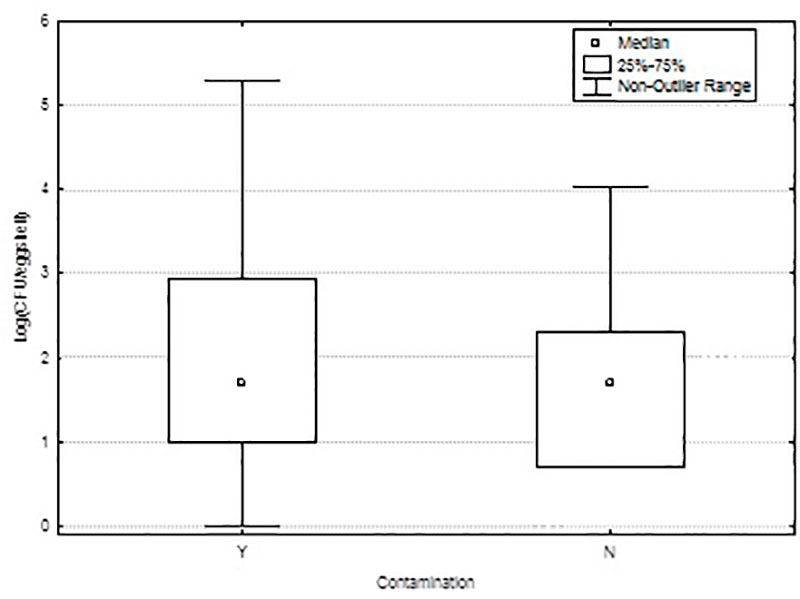
Figure 3: Total count of inoculated 7 species on the eggshell of contaminated whole eggs (Y) and non-contaminated whole eggs (N) considering all strains (De Reu et al. 2006c).
Influence of the housing system on the bacterial contamination of table eggs
With the introduction of alternative housing systems for laying hens in the EU, from 2006 until 2012 research focused on the bacterial contamination of table eggs, e.g. eggshell and egg content contamination. Contamination of eggshells with aerobic bacteria is generally comparable for nest eggs derived from non-cage systems compared to furnished cage nest eggs or conventional cages. Studies indicate a higher bacterial load for outside nest eggs as well from furnished cages as from non-cage systems. The major differences found by De Reu et al. (2005) in experimental studies between furnished cage and non-cage systems are less pronounced under commercial conditions (figure 4) (De Reu et al. 2009). The effect of housing system on eggshell contamination with specific groups of bacteria is variable (De Reu et al. 2009). Limited information is available on the influence of housing system on egg content contamination. Research of De Reu et al. (2006e, 2007, 2008) does not indicate large differences in egg content contamination between eggs from cage- and non-cage systems (ignoring outside nest and floor eggs).
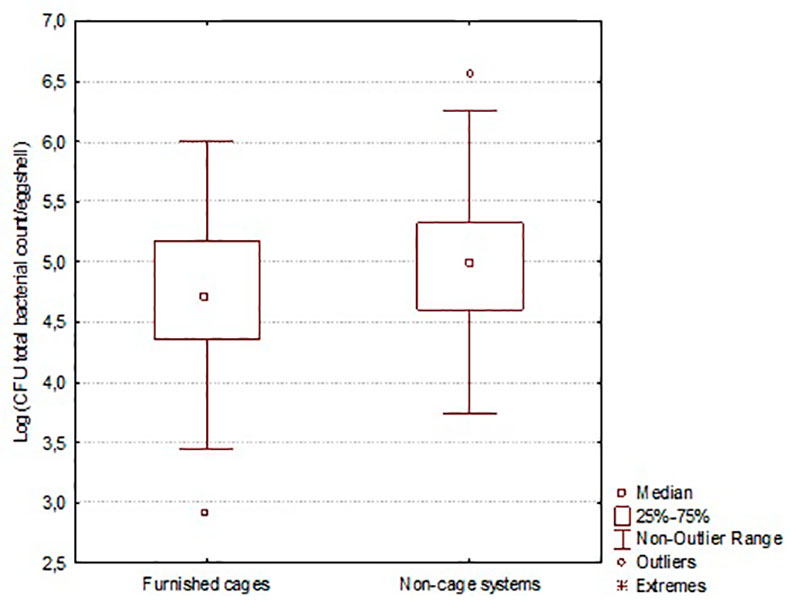
Figure 4: Eggshell contamination with total count of aerobic bacteria in furnished cages (7 flocks) compared to non-cage systems (6 flocks) (De Reu et al. 2009)
Studies also show that it is highly unlikely that a move from conventional cages to alternative cage systems and non-cage housing systems will result in an increase in Salmonella infection and shedding, rather the opposite is expected (Van Hoorebeke et al. 2010).
Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella
Enteritidis Trans-shell infection routes and whole egg contamination of 7 phylogenetically divers bacterial strains showed interesting results. Eggshell penetration was correlated with various eggshell characteristics and the identity of strains. Contrary to the cuticle deposition (figure 5), the shell surface area, shell thickness and number of pores did not influence the eggshell penetration (De Reu et al. 2006c).
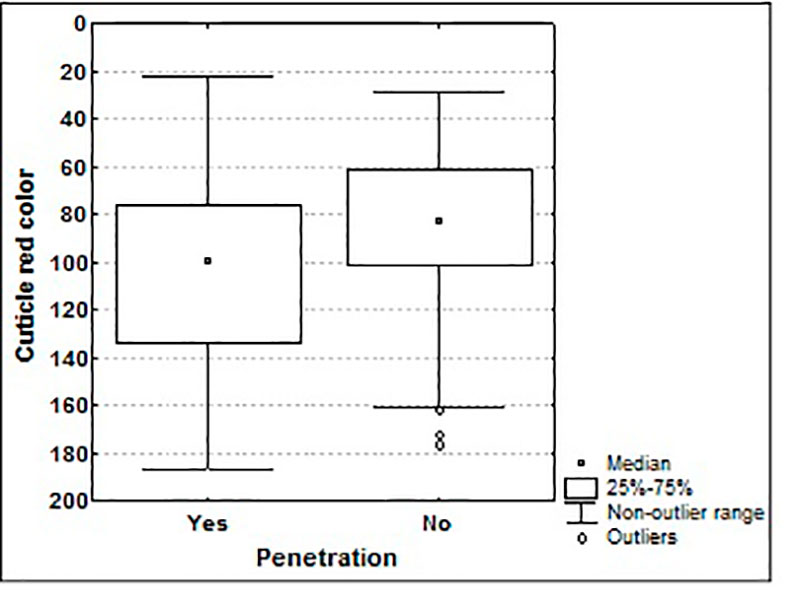
Figure 5: Influence of cuticle deposition on eggshell penetration of 7 bacterial species (lower red color = more cuticle deposition) (De Reu et al. 2006c)
The results indicate that Gram-negative, motile and non-clustering bacteria penetrated the eggshell most frequently (figure 6a); Pseudomonas sp. and Alcaligenes sp. (58%) were primary invaders followed by SE. In comparison with the non-Salmonella strains, SE was a primary invader of whole eggs suggesting not only the eggshell penetration capability of SE as motile bacterium but also the capability to survive the anti-microbial properties of the albumen (figure 6b). Egg related SE strains had no special capacity to contaminate whole eggs compared to other Salmonella serovars. Penetrated eggshells and contaminated whole eggs showed a significant higher bacterial eggshell contamination (De Reu et al. 2006c).
Those findings are not always confirmed by other researchers. The defence of the cuticular layer has for example questioned by Nascimento et al. (1992) and Messens et al. (2005a) using agar-filled eggs. Other researchers found that SE had special capabilities to survive in the albumen compared to other Salmonella serovars. On the other hand attempts made by several workers to correlate eggshell porosity with bacterial penetration gave varying results.
The most important contribution of the shell is to provide a mechanical protection (Board and Tranter 1995). The shell also forms a physical barrier against external microbial contamination. De Reu et al. (2006c) found no relationship between shell thickness and the likelihood of bacteria including SE to penetrate the eggshell. The shell membranes are very effective barriers to bacterial penetration and at the same time have antibacterial properties.
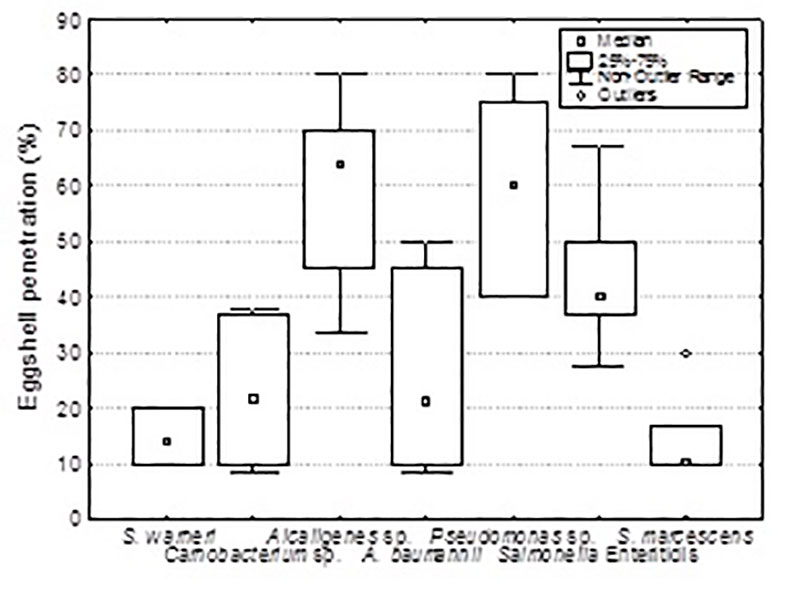
Figure 6a: Percentage of eggshell penetration (agar method) for each individual bacterial strains
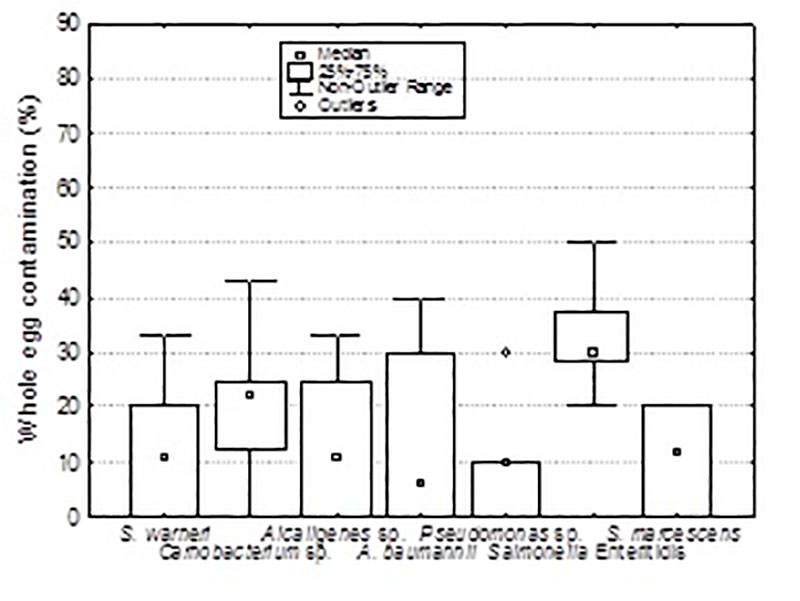
Figure 6b: Percentage whole egg contamination (intact egg method) for each individual bacterial strains (De Reu et al. 2006c)
Influence of sweating (condensate) and hen age
During storage and transport until consumption excessive temperature fluctuations should be avoided because this provokes water condensation and subsequently microbial growth and possible eggshell penetration. De Reu et al. (2006b) found a short sweating period of the egg influences the eggshell penetration (agar method) but had no impact on the egg content contamination (intact egg method). In the study condensation on the eggshell encouraged the bacterial eggshell penetration with SE but it had a smaller and not significant impact on the whole egg contamination. The higher survivals of the pathogen on the eggshells of agar-filled eggs with condensate might explain the higher penetration of those eggshells. The low impact of condensation on the whole egg contamination can be explained by the equal survival of SE on the eggshell of whole eggs with and without condensation, and by the antimicrobial defences of the albumen. The higher whole egg contamination found at the end of lay compared to the previous hen ages showed older eggs might be more susceptible for egg content contamination. There are many possible reasons for the higher susceptibility for bacterial infection of eggs from older hens: cuticle deposition decreases, decrease in shell quality, less antimicrobial properties of egg albumen, … Although the various research results sometimes contradict each other.
Salmonella contamination and Salmonella control programs
SE is considered the only pathogen currently posing a major risk of egg-borne diseases in the European Union. Namely, eggs and egg products are most incriminated in outbreaks caused by SE but in few occasions they are also susceptible to contamination with other pathogens, such as B. cereus, Staphylococcus aureus, Listeria monocytogenes and Campylobacter (EFSA 2014). Much of the research on eggshell and egg content contamination focuses on Salmonella, and especially SE. Observed Salmonella prevalence on the eggshell and in the egg content vary, depending on the fact whether investigations were based on randomly sampled table eggs or on eggs from naturally infected hens. Dewaele et al. (2012) found on naturally infected SE farms 0.18 – 1.8% contaminated eggshells and 0.04% – 0.4% egg contents. In 2003, the European Commission issued Regulation No. 2160/2003, requiring member states to take effective measures to detect and control Salmonella and other zoonotic agents, not only at the level of primary production of animals, but also at other stages in the food production chain. This legislation specified general requirements to establish a national control program (NCP) for Salmonella. A subsequent Directive (2003/99/EC) aimed at ensuring that zoonoses, zoonotic agents and their antimicrobial resistance are properly monitored, and that food-borne outbreaks are properly investigated. Later, minimal demands for NCPs in laying hen holdings were stated in Regulations No. 1168/2006 and 1177/2006 as amended by Regulation No. 517/2011. The application of Salmonella control programmes at EU level has led to a clear decrease in the incidence of SE infection in laying hen flocks, and of human infections due to this serovar.
Influence of storage temperature and time
De Reu et al. (2008) found the level of eggshell contamination by total aerobic flora and Gram-negative bacteria was shown to decrease over a storage period of nine days or more in both non-refrigerated and refrigerated eggs. The survival of Salmonella on egg shells is possible and is enhanced by low temperature. Survival is inversely related to both storage temperature and relative humidity (Humphrey, 1994; Messens et al. 2006). In egg albumen SE grows slowly and only to a limited extent because the albumen contains multiple antimicrobial components, including bacterial cell wall and DNA damage (Gantois et al., 2009). At less than 8°C Salmonella is unable to grow in albumen (EFSA 2014) and a bactericidal effect is observed at 4°C in a few days. These findings show that cooling of eggs is appropriate to reduce bacterial infection and outgrowth with Salmonella and other spoiling organisms.

Conclusions
It is clear that several factors play a role in spoilage and Salmonella contamination of table eggs. But under healthy breeding conditions an egg’s content is generally sterile just after laying and good handling and preservation further in the agri-food chain will certainly reduce the risk for microbiological contamination to an exception.
References
BOARD, R. G. and TRANTER, H. S. (1995). The microbiology of eggs, in. W. J. Stadelman, Cotterill, O.J. (Eds.) Egg science and technology. New York, Food Products Press – The Haworth Press, Inc.: 81-104.DE REU, K., GRIJSPEERDT, K., HEYNDRICKX, M., ZOONS, J., DE BAERE, K., UYTTENDAELE, M., DEBEVERE, J. and HERMAN, L. (2005). Bacterial eggshell contamination in conventional cages, furnished cages and aviary housing systems for laying hens. British Poultry Science 46: 149-155.
DE REU, K. (2006a) Bacteriological contamination and infection of shell eggs in the production chain. Ph. D. Thesis. University of Ghent.
DE REU, K., GRIJSPEERDT, K., HEYNDRICKX, M., MESSENS, W., UYTTENDAELE, M., DEBEVERE, J. and HERMAN, L. (2006b). Influence of eggshell condensation on eggshell penetration and whole egg contamination with Salmonella enterica serovar Enteritidis. Journal of Food Protection 69: 1539-1545.
DE REU, K., GRIJSPEERDT, K., MESSENS, W., HEYNDRICKX, M., UYTTENDAELE, M., DEBEVERE, J. and HERMAN, L. (2006c). Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella Enteritidis. International Journal of Food Microbiology 112: 253-260.
DE REU, K., GRIJSPEERDT, K., HERMAN, L., HEYNDRICKX, M., UYTTENDAELE, M., DEBEVERE, J., PUTIRULAN, F. F. and BOLDER, N. M. (2006d). The effect of a commercial UV disinfection system on the bacterial load of shell eggs. Letters in Applied Microbiology 42: 144-148
DE REU, K., HEYNDRICKX, M., GRIJSPEERDT, K., RODENBURG, B., TUYTTENS, F., UYTTENDAELE, M., DEBEVERE, J. and HERMAN, L. (2006e). Assessment of the vertical and horizontal aerobic bacterial infection of shell eggs. World’s Poultry Science Journal 62 (supplement): 564.
DE REU, K., HEYNDRICKX, M., GRIJSPREEDT, K., RODENBURG, B., TUYTTENS, F., UYTTENDAELE, M., DEBEVERE, J. and HERMAN, L. (2007). Estimation of the vertical and horizontal bacterial infection of hen’s table eggs, XVIII European symposium on the quality of poultry meat & XII European symposium on the quality of eggs and egg products – Conference proceedings, Prague: 55-56.
DE REU, K., MESSENS W., HEYNDRICKX M., RODENBURG B., UYTTENDAELE M. and HERMAN L. (2008). Bacterial contamination of table eggs and the influence of housing systems. World’s Poultry Science Journal 64: p. 5-19.
DE REU, K., RODENBURG, B., GRIJSPEERDT, K., MESSENS, W., HEYNDRICKX, M., TUYTTENS, F., SONCK, B., ZOONS, J. and HERMAN, L. (2009). Bacteriological contamination, dirt, and cracks of eggshells in furnished cages and noncage systems for laying hens: An international on-farm comparison. Poultry Science 88: p. 2442-2448.
DEWAELE, I. , VAN MEIRHAEGHE, H., RASSCHAERT, G., VANROBAEYS, M., DE GRAEF, E., HERMAN, L., DUCATELLE, R., HEYNDRICKX, M. and DE REU, K. (2012). Persistent Salmonella Enteritidis environmental contamination on layer farms in the context of an implemented national control program with obligatory vaccination. Poultry Science 91: p. 282-291.
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014. Scientific Opinion on the public health risks of table eggs due to deterioration and development of pathogens. EFSA Journal 2014; 12(7):3782, 147 pp. doi:10.2903/j.efsa.2014.3782
GANTOIS, I, DUCATELLE, R, PASMANS, F, HAESEBROUCK, F, GAST, R, HUMPHREY, TJ and VAN IMMERSEEL, F (2009) Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol Rev. 33: 718-738
HUMPHREY, T. J. (1994a). Contamination of egg shell and contents with Salmonella enteritidis: a review. International Journal of Food Microbiology 21: 31-40.
MAYES, F. J. and TAKEBALLI, M. A. (1983). Microbial contamination of the hen’s egg: A review. Journal of Food Protection 46: 1092-1098.
MESSENS, W., GRIJSPEERDT, K. and HERMAN, L. (2005). Eggshell characteristics and penetration by Salmonella enterica serovar Enteritidis through the production period of a layer flock. British Poultry Science 46: 694-700.
NASCIMENTO, V. P., CRANSTOUN, S. and SOLOMON, S. E. (1992). Relationship between shell structure and movement of Salmonella enteritidis across the eggshell wall. British Poultry Science 33: 37-48.
VANHOOREBEKE, S., VAN IMMERSEEL, F., SCHULZ, J., HARTUNG, J., HARISBERGER, M., BARCO, L., RICCI, A., THEODOROPOULOS, G., XYLOURI, E., DE VYLDER, J., DUCATELLE, R., HAESEBROUCK, F., PASMANS, F., DE KRUIF, A. and DEWULF, J. (2010). Determination of the within flock prevalence and identification of risk factors for Salmonella infections in laying hen flocks housed in conventional and alternative systems. Preventive Veterinary Medicine 94, 94 – 100
