Summary
Although shell color says nothing about the nutritional value of the egg, a uniformly dark brown shell
color is considered as one of the important traits for external quality. The heritability of shell color is
moderately high (h2 = 0.35 to 0.45), which allows the breeding companies to improve this trait through
selection. With the help of a reflectometer the shell color can be measured on a continuous objective
scale, which correlates well with a subjective score, and which allows to select for this trait and to
compare values across locations and years and between different ages of the same flock.
Shell color tends to become lighter as hens age, but close genetic correlations between measurements
at different ages suggests that hens which lay eggs with a dark shell at peak production will also tend
to lay dark colored eggs at the end of the cycle. Eggs with darker shells tend to have more and/or
larger blood and meat spots, as well as a higher incidence of speckles. Exclusive reliance on the
measurements with the Minolta device could therefore lead to undesirable correlated effects, unless
they are monitored and compensated by using additional data.
Finally, the natural shine of the egg shell was measured comparing the reflection from different angles
with the help of a spectrophotometer. It was found to be a moderately heritable trait (h2 = 0.15 to
0.39), which could be used in addition to shell strength and shell color to select for attractive shell
eggs.

Introduction
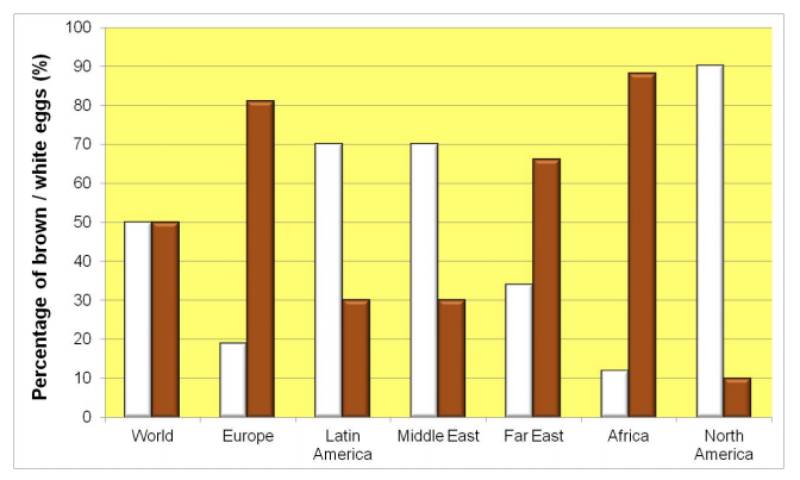
First quality eggs must have first quality shells, i.e. they must be clean and have strong shells to withstand handling and transportation. In addition to these primary shell quality criteria and adequate egg weight, a uniform and attractive white or brown shell color is very important to the consumer. The consumer assesses the quality of an egg according to his specific subjective demands, and one of these demands is clearly eggshell color. The proportion of white and brown eggs consumed in the world is roughly 50:50, with significant differences between continents in preferred shell color (figure 1).Figure 1: Estimated percentage of brown and white eggs worldwide.
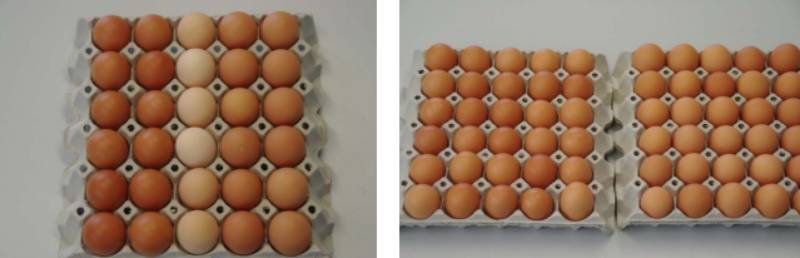
Shell color is not an indication of internal egg quality and says nothing about the nutritive value or the quality of the egg (Flock et al., 2007). However, many consumers who prefer brown eggs, also pay attention to intensity and uniformity of color, and pale or unevenly colored eggs may be rejected. Clearly, reduced variability of eggshell color improves the presentation of eggs at point of sale (Figure 2). Pigments produced in the uterus at the time of shell formation are responsible for egg shell color. The brown color of eggshells is mainly caused by the pigments protoporphyrin-IX, biliverding-IX and Zinc chelate, which is distributed throughout the entire shell. Pigment is added to the shell quite late in the shell formation process. Therefore, problems with poor pigmentation may occur if the egg is laid prematurely (Nys et al., 1991). Furthermore, the whitening of the shell could be due to an impairment of cut-off mechanisms toward the end of calcification or delays in the oviposition time, rather than changes in the amount of pigment deposited. The final process in shell formation is the deposition of the cuticle. Some authors have argued that eggs with poor shell pigmentation may lack the cuticle layer or parts thereof.
Figure 2. Range of shell color which may be seen in unselected flocks of mixed origin (left)
vs. typical variation of shell color in flocks with good uniformity
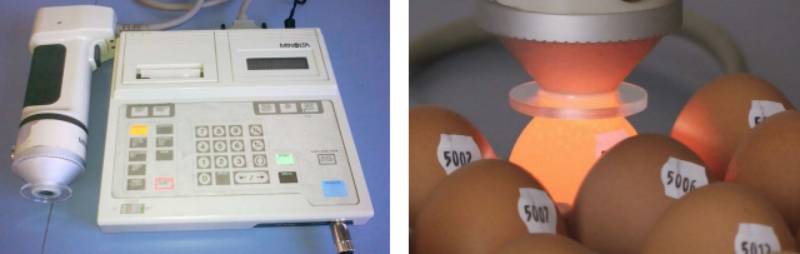
Color determination with the L*a*b* Color System
The color of an object is determined by pigments. These are chemicals which create a given color by subtracting parts of the spectrum of the incident light. The remaining light is reflected and this gives the object its color (Konica Minolta, 2012). Color is a matter of perception and subjective interpretation of the person looking at the object. When colors are classified, they can be expressed in terms of their lightness (brightness), hue (color) and saturation (vividness). Using the Minolta device (Reflectometer CR 300, figure 3), the color of each individual egg can be objectively determined by the following three parameters:- L*: lightness (value between 0= black and 100=white)
- a*: hue as a function of the red-green scale (<0 >0 = red)
- b*: hue as a function of the blue-yellow scale (<0 >0 = yellow)
Figure 3: Reflectometer used to measure eggshell color
The L*a*b* Color System (also known as the CIELab System) was introduced in 1976 and is today one of the most commonly used systems for measuring object colors in many fields. With these three parameters, shell color can be described objectively within the color spectrum. As a* and b* increase (in absolute value) the saturation of the color increases.
In addition to subjective scoring of shell color, Lohmann Tierzucht GmbH started in the early 1990s to measure egg shell color in large numbers of pedigreed brown-egg layers with the Minolta reflectometer to speed up selection for dark shell color. Comparisons of subjective scores with the three reflectometer readings confirmed that the most attractive eggs had low L* values and high positive a* and b* values (Förster et al., 1996). These authors defined “good eggshell color” with the following reference values: L* = 60, a* = 20 and b* = 30.
A shell color index based on the three color parameters is routinely calculated with the formula SCI = L* – a* – b*, lower values indicating darker shell color. While subjective scores depend on light sources in the observation room and preferences of individual graders, the objective parameters are measured on a continuous scale and can be compared across locations and years and between different ages of the same flock. More important from a breeder’s point of view is that the variation within a pedigreed flock can be analyzed as a normally distributed quantitative trait and used for systematic selection in the direction of consumer preferences.
Eggshell Color as a breeding goal
Commercial brown-egg lines have been selected for attractive dark brown shells for many years, based on subjective evaluation and quantitative measurement of shell color. Moderate values of heritability for eggshell color reported in the literature range from 0.46 to 0.50 (Förster et al., 1996, Zhang et al. 2005; Flock et al. 2007). As indicated by the relatively high heritability, there is considerable variation in shell color among families and individual hens within a line. Since the breeding goal is to select for dark brown eggshells, individuals with a breeding value for sub-standard shell color are unlikely to be selected – unless they are outstanding in most other traits. The overall breeding goal is focused on a high number of “saleable” eggs, i.e. to get selected, a candidate must have positive breeding values for both egg number and egg quality, while more eggs with undesirable shell quality are least desired.Table 1: Eggshell color index of different commercial brown-egg strain crosses (Random
Sample Test Ustrasice, average of conventional and enriched cages).
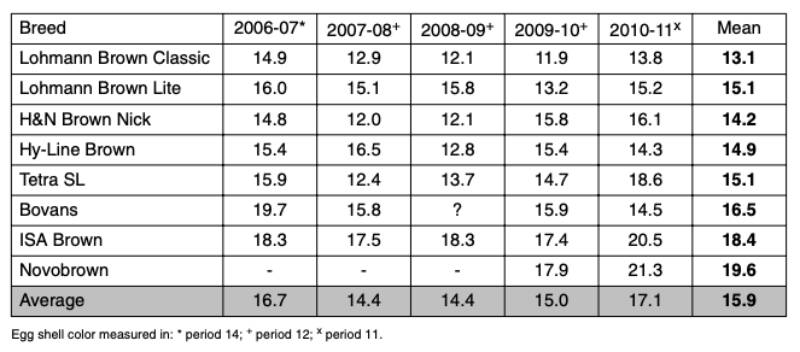
The variation from test to test (shown in table 1) indicates environmental effects, even in a standardized test environment with uniform conditions, and random variation due to small groups of birds.
Effect of hen age on eggshell color
It has been reported that older hens tend to lay larger eggs with lighter shell color. This is because the quantity of pigments deposited on the shell surface does not increase in proportion to egg size. Hence, the pigments of brown eggshells are deposited over a larger surface area as the hen ages and lays larger eggs (Solomon, 1997). Cavero et al. (2010) studied the influence of hen age on eggshell color. Data for the analysis were obtained from a flock of 4,400 Rhode Island Red and a flock of 4,743 White Rock pure-line hens in single cages on a breeding farm. As shown in table 2 the eggshells became significantly lighter with increasing age in the RIR line, whereas the WR line still had excellent shell color at 60 weeks of age. The L* and b* values were relatively constant throughout time, whereas the a* values decreased considerably, especially in the RIR line. The comparison of the two breeds suggests that persistency of eggshell color is also a realistic goal for within-line selection.Table 2: Change in eggshell color with increasing age (Cavero et al., 2010)
The genetic parameters were found to be very similar in both lines. Table 3 shows the estimated heritabilities and genetic correlations for the Rhode Island Red line.
Table 3: Estimated heritabilities and genetic correlations at different ages
(Cavero et al., 2010)
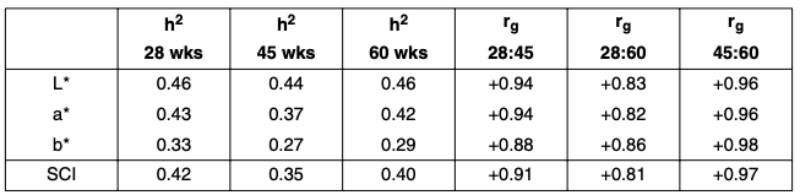
The heritability estimates of all color parameters were similar at the three ages and ranged from h2 = 0.27 to h2 = 0.46. Genetic correlations among the three parameters are not reported in detail here. The correlation between L* (lightness) and a* (red) was consistently highly negative in both lines (rg = -0.82 to -0.97), whereas the correlations between b* (yellow) and L* and a* varied strongly between the lines and age at measurement (rg = -0.01 to -0.73 and rg = +0.02 to +0.71 respectively). The close genetic correlations between measurements at the different ages suggests that hens which lay eggs with a dark shell color at peak production will also tend to lay dark colored eggs at the end of the cycle, indicating a strong genetic component for general shell pigmentation. If additional measurements at the end of the laying period add little to the accuracy of breeding value estimation, measuring eggshell color at an intermediate age should be sufficient to monitor and further improve lifetime eggshell color.
Relationship between shell color and other important traits
The traits included in this study were egg production in three phases: from 20 to 28 weeks (EP1), from 28 to 48 weeks (EP2) and from 48 to 72 weeks (EP3); egg weight (EW); shell strength (SS); blood and meat spots (BMS); and dark “speckles” on the eggshell (SPE). BMS and SPE are subjec tive scores (between 1 and 9), higher values indicating lower incidence and smaller size of the spots, i.e. desired direction from a breeding point of view.Table 4: Genetic correlations between shell color (SCI) and other traits.
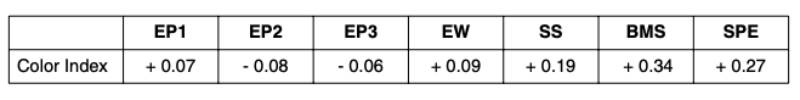
Negative genetic correlations indicate lower SCI and better shell color, i.e. they are desired from a breeding point of view. The genetic correlation between shell color index and egg production is not significantly different from zero, and the slightly positive correlation with egg weight is expected as explained above. The positive genetic correlation with shell strength is small, but needs to be observed while selecting for both strong shells and attractive shell color. The significantly positive correlations with scores for speckles and blood and meat spots are in accordance with Förster et al., (1996), who found that eggs with darker shells tend to have more and/or larger blood and meat spots. In fact, some of the blood or meat spots might result from the deposition of color pigments into the subsequent egg. The positive correlation between shell color and incidence of speckles confirms the expectation that the Minolta device reads the dark spots on brown eggs as “darker” brown. Some consumers actually like speckled eggs, but to avoid an increased incidence of speckles (Arango et al., 2006) and achieve uniform dark brown shell color, we will continue to score the incidence of speckles in addition to taking the objective color measurements, to assure that we are selecting in the desired direction.
New ideas to describe and improve “attractiveness” of eggshell color
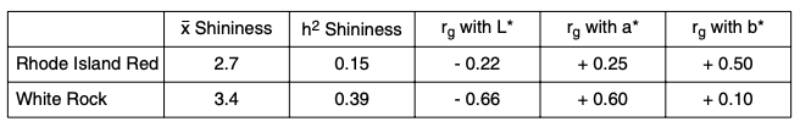
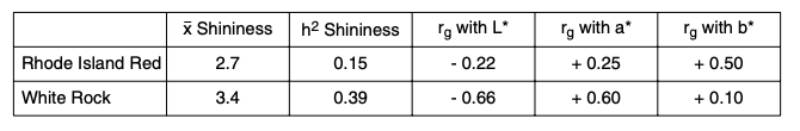
Some eggs look more attractive than others, because they have a natural “shine” as if they were washed and oiled. This phenomenon can be observed in both white and brown eggs with different frequency. In Europe and other countries where washing and oiling of eggs is not permitted, it would be interesting to know whether the shine of the eggs is a heritable trait which could be used to improve the attractiveness of shell eggs at point of purchase. We have tested a new device (Spectrophotometer Minolta CM 600d) along with routine measurements of egg shell color parameters (L*a*b*). Data for shininess of eggshells were collected for two brownegg pure lines to estimate genetic parameters for this new characteristic. Shininess is measured by comparing the reflection from different angles. An eggshell with a value of 0 has no shine and is completely matt, and the higher the measured value, the shinier is the eggshell. In this study the shininess of the eggs varied between 0 and 14, with an average of 2.6 and standard deviation 2.1. The shininess was lower in the Rhode Island Red line, compared to the White Rock line. As shown in table 5, this trait had a moderate heritability and desirable genetic correlations with all three color parameters: numerically positive with a* and b* and negative with L*.Table 5: Heritability of the egg shell shininess and its genetic correlation with three color
parameters
Perhaps even more interesting than aesthetic considerations would be to find a positive relation between the shininess of the egg shell and increased protection against pathogen penetration. It seems reasonable to expect that eggs exhibiting a brilliant shine are more likely to have an intact cuticle than eggs with matt appearance.
The cuticle is a proteinaceous layer, which coats the outside of the egg and plays a major role in preventing bacteria from entering the egg. In a recent study using a dye combined with reflectance spectrometry the preliminary results suggest that the cuticle staining is a moderately heritable trait with h2 = 0.27 (Bain et al., 2009). An intact and functioning cuticle could play a key role in selection strategies to support food safety efforts. The measurement cited above is quite costly and time consuming, so it would be interesting to find an indirect measurement which can be recorded with less cost on many eggs. In a preliminary test with a small number of eggs no difference could be found between shiny and dull eggs in relation to the presence of the cuticle (Bain 2011, personal communication).
Conclusions
Improving eggshell quality is a significant objective for breeders to satisfy consumer preferences. The heritability of shell color is moderately high (h2 = 0.35 to 0.45), which allows the breeding companies to achieve further genetic improvement in commercial layers. Shell color in brown eggs tends to deteriorate toward the end of the laying cycle, but a close genetic correlation at different ages assures that early measurements will also improve life time shell color. There are apparently no serious antagonistic correlations with other traits except speckles on the shells and blood and meat spots in the eggs. Therefore, selection for improved eggshell color in brown-egg layers has to keep these traits in mind to avoid undesirable correlated response. The natural shine some unwashed and unoiled eggs exhibit appears to be a moderately heritable trait, which could be used in addition to shell strength and shell color to select for attractive shell eggs. Preliminary studies with special equipment have not confirmed the assumption that the shine on eggs also reflects an intact cuticula, which would be highly desirable in the context of food safety. While eggshell quality is receiving a lot of attention in genetic selection programs, egg producers should be aware of all non-genetic factors which must to be controlled to satisfy high customer expectations in oversupplied markets. In addition to high quality feed and water, effective control of diseases and air quality, monitoring the functioning of all equipment, special attention must be paid to frequent egg collection and egg storage under optimal conditions.References
ARANGO J.A., P. SETTAR, J.A. ARTHUR and N.P. O’SULLIVAN (2006): Relationship between shell color and incidence of speckles in brown egg lines. Proc. XIIth European Poultry Conference, Verona, Italy.BAIN, M.M., K. MCDADE, M. SCHMUTZ, R. PREISINGER, D. WADDINGTON and I.C. DUNN (2009): The deposition of the cuticle on laying hens eggs is a moderately heritable trait. British Poultry Abstracts 5, 17.
CAVERO, D., M. SCHMUTZ and R. PREISINGER (2010): Breeding for optimal eggshell color in brown-egg layers. Proc. XIIIth European Poultry Conference, Tours, France.
FLOCK, D.K., M. SCHMUTZ and R. PREISINGER (2007): Optimierung der Eiqualität aus züchterischer Sicht. Züchtungskunde, 79: 309-319.
FÖRSTER, A., D. JAENECKE, M. WITTMANN, D.K. FLOCK and M. KREUZER (1996): Use of fotometrically determined shell color parameters as selection criteria for marketable brown shelled eggs. Archiv für Geflügelkunde, 60: 1-6.
KONICA MINOLTA (2012): The language of light – From perception to instrumentation.
NYS, Y., J. ZAWADZKI, J. GAULTRON and A. D. MILLS (1991): Whitening of brown-shelled eggs: Mineral composition of uterine fluid and rate of protoporphyrin deposition. Poultry Science 70: 1236-1245.
ODABASI, A. Z., R. D. MILES, M. O. BALABAN and K. M. PORTIER (2007): Changes in brown eggshell color as the hen ages. Poultry Science 86: 356-363.
SOLOMON, S. E. (1997): Egg and eggshell quality. Iowa State University Press, Ames.
ZHANG L.-C., Z.-H. NING, G.-Y. XU, Z.-C. HOU and N. YANG (2005): Heritabilities and genetic and phenotypic correlations of egg quality traits in brown-egg dwarf layers. Poultry Science 84: 1209-1213.
Zusammenfassung
Attraktive Schale als ZuchtzielObwohl die Schalenfarbe nichts über den Nährwert eines Eies aussagt, ist eine einheitlich dunkelbraune Eischalenfarbe ein wichtiges äußeres Eiqualitätsmerkmal. Eine hohe Heritabilität für die Eischalenfarbe (h2 = 0,35 bis 0,45) ermöglicht eine züchterische Verbesserung dieses Merkmals durch Selektion. Mit Hilfe eines Reflektometers kann die Farbe der Eischale objektiv, in enger Beziehung zur subjektiven Farbbeurteilung, gemessen werden. Diese erfassten Farbwerte ermöglichen einen objektiven Vergleich von Eiern verschiedener Betriebe und Jahre, sowie unterschiedlicher Altersklassen einer Herde. Die positive genetische Korrelation zwischen der Eischalenfarbe in unterschiedlichen Altersklassen zeigt, dass die Hennen welche in der Produktionsspitze Eier mit einer dunkleren Eischalenfarbe legen, auch am Produktionsende noch dunklere Eier haben, obwohl die Eischalenfarbe generell mit zunehmendem Alter der Hennen heller wird. Ungünstig sind die genetischen Beziehungen zur Schalenstabilität sowie zur Anzahl/Größe von Blut-und Fleischflecken und dem Auftreten von Sprenkeln. Diese Zusammenhänge müssen bei der Selektion berücksichtigt werden, um unerwünschte Veränderungen bei diesen Merkmalen zu vermeiden. Weiterhin wurde der natürlich Glanz der Eischale durch einen Vergleich der Reflektion aus verschiedenen Winkeln mit einem Photospektrometer gemessen. Für dieses Merkmal wurde eine moderate Heritabilität von h2 = 0,15 bis 0,39 geschätzt, und es kann als zusätzliches Kriterium genutzt werden, um auf noch attraktivere Eischalenfarbe zu selektieren.
Подпишитесь на нашу рассылку
И узнайте обо всех последних новостях индустрии.