Summary
Salmonella is one of the major sources of food-borne disease in humans. Contaminated eggs still
cause high numbers of cases of human salmonellosis. Vaccination of poultry flocks against Salmonella
Enteritidis and Salmonella Typhimurium is an important part of the biosecurity and hygiene programs
to prevent infection in the first place. The new combined homologous vaccine tested in this study was
proven to be safe and efficacious after challenge infection with virulent strains of both serovars.
Introduction
Even after more than a decade of combating Salmonella infections, this organism still represents an
important cause of human disease (EFSA, 2009, Newell et al., 2010). Recent studies estimate 80.3
million annual cases of food-borne disease related to Salmonella worldwide (Majowicz et al., 2010).
Within the European Union Salmonella is the second most important cause of food-borne infections
(EFSA, 2007) with Salmonella Enteriditis still representing the most commonly isolated Salmonella
serovar in human salmonellosis (EFSA, 2007; WHO, 2006). Even today contaminated eggs from
infected layers remain the major source of Salmonella Enteriditis infection (Delmas et al., 2006; EFSA,
2007; Korsgaard et al., 2009; Stevens et al., 2009). As a consequence all member states of the
European Union have to implement Regulation EC No. 2160 from 2003 on a national basis in order
to control Salmonella and other zoonotic agents of significance for public health in farm animals. The
prevention of Salmonella infections in laying hens and the control of the pathogen at farm level is the
key to producing safe egg products and to being in line with Regulation EC No. 2160/2003. As
described recently by Carrique-Mas and Davies (2008) the member states of the EU have to invest
more in the prevention, detection and control of Salmonella infections in laying hens.
Vaccination plays an important role in the overall biosecurity system on chicken farms to prevent
Salmonella infections (Temelli et al., 2010). When vaccination first arose as a method of combating
this organism, inactivated vaccines were developed by various companies. Due to many reasons,
such as ease of application, animal welfare, and especially efficacy, attenuated live vaccines entered
the market with great success a short time later. These live, attenuated vaccines were homologous
vaccines against either Salmonella Enteritidis or Salmonella Typhimurium. Scientific evidence shows
that serovar overlapping effects exist, but homologous vaccines offer the best protection against
infection (Springer et al., 2000; Martin et al., 1996; Chacana et al., 2006).
In this paper results on the safety and efficacy of a new combined homologous vaccine against
Salmonella Enteritidis and Salmonella Typhimurium (Lohmann testing vaccine) are discussed.
Safety of the vaccine
The Lohmann testing vaccine is safe for day-old Specific Pathogen Free (SPF) chicks, the most
susceptible chickens for infection with Salmonella, when administered with a single, repeated or 10-
fold dose. The dissemination of the vaccine strains is limited and the strains do not persist in internal
organs for a long period of time. The vaccine is also not transmitted on or into the eggs in vaccinated
birds.
Safety trials with day-old Peking ducklings showed that oral application of the vaccine is completely
safe to use. The Lohmann testing vaccine also represents no health hazard to turkeys or any other
tested species (Lohmann Animal Health, data on file).
Oral application via drinking water of the Lohmann testing vaccine to 25.000 day-old commercial
layers under field conditions was proven to be safe. Repeated vaccination on day one, week 6 and week
16 of life was observed under field conditions and no safety issues caused by the vaccination were
observed (Table 1).
Table 1: Safety of repeated oral application of the Lohmann testing vaccine under field
conditions in two flocks, each with 12.500 commercial layers (Hy-Line Brown)
(Lohmann Animal Health, data on file)

Table 2: Vaccination scheme
Efficacy and duration of immunity
An optimum vaccine should protect against Salmonella infection throughout the laying period. In order
to test efficacy of the immune response at the beginning and at the end of the laying period, SPF
(Lohmann Selected Leghorn) birds were vaccinated orally either with a minimum dose of the Lohmann
testing vaccine, AviPro® Salmonella Vac E or AviPro® Salmonella Vac T on the first day of life, in
week 6 and in week 16 (Table 2). Vaccinated birds were kept throughout the laying period.
At the beginning of production (week 21 or 22 of life) birds from each group were challenged orally with
either 2 x 109 cfu of Salmonella Enteritidis Nalres or 3 x 109 cfu of Salmonella Typhimurium K284/93
Nalres per bird. Seven days post challenge the caeca and liver of all birds were investigated
bacteriologically for the presence of the challenge strain. The liver and caeca of the chickens vaccinated
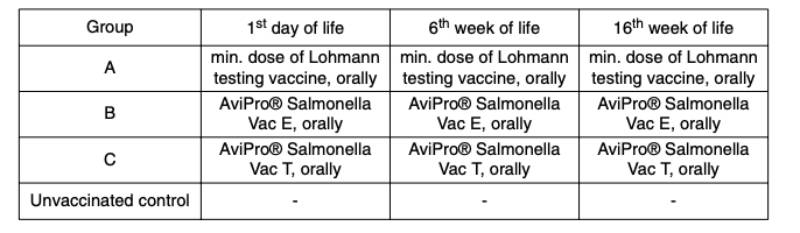
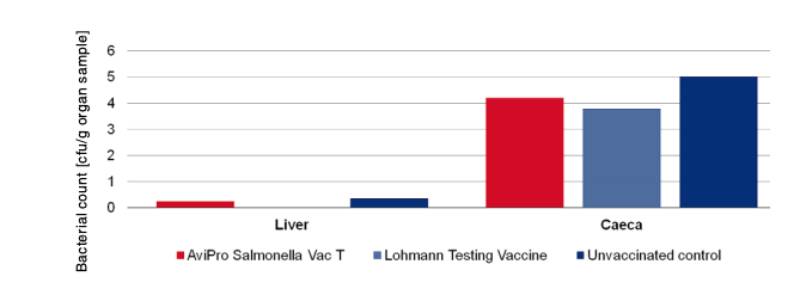
with either the monovalent vaccines (AviPro® Salmonella Vac E (Figure 1) or AviPro® Salmonella
Vac T (Figure 2)) or Lohmann Testing Vaccine showed a reduction in their colonisation by the Salmonella
field strains as compared to unvaccinated chickens.
At an age of 68 weeks 10 birds were challenged with 2 x 109 cfu of Salmonella Enteritidis Nalres per
bird and analysed in comparison to an unvaccinated, challenged control group of the same hatch
that was kept under the same conditions. Seven days post challenge the caeca and liver of all birds
were examined bacteriologically for the presence of the challenge strain. The unvaccinated control
birds had diarrhoea starting 4 days post challenge. The vaccinated birds showed no clinical signs.
Bacteriological analysis revealed that the titre of the virulent strain Salmonella Enteritidis Nalres was
significantly reduced in the liver and caeca of vaccinated birds as compared to organs of unvaccinated
control birds (Figure 3). Persistence of the challenge strain in the liver was completely inhibited in
the vaccinated birds.
Figure 1: Persistence of the challenge strain Salmonella Enteritidis Nalres in internal organs
7 days post infection in week 21
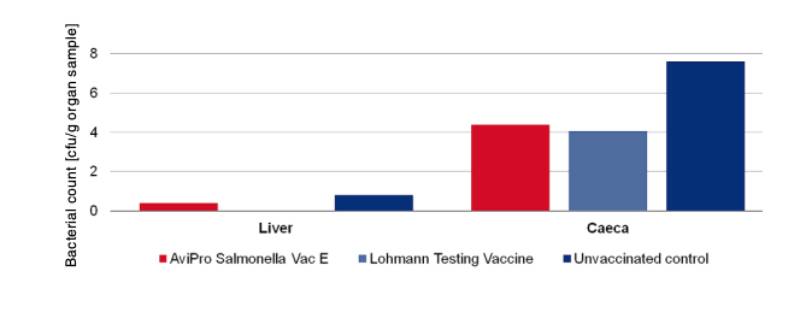
Figure 2: Persistence of the challenge strain Salmonella Typhimurium K284/93 Nalres in
internal organs 7 days post infection in week 22
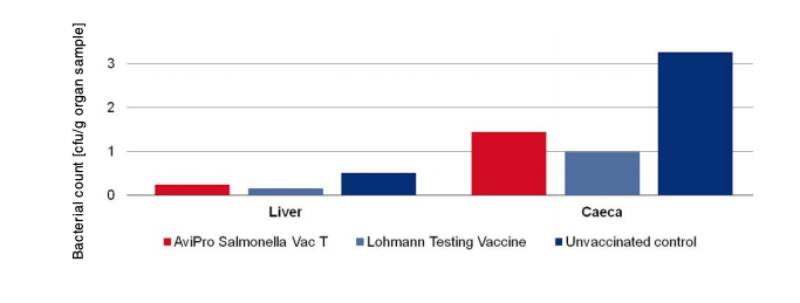
Figure 3: Persistence of the challenge strain Salmonella Enteritidis Nalres in internal organs
7 days post infection in week 68
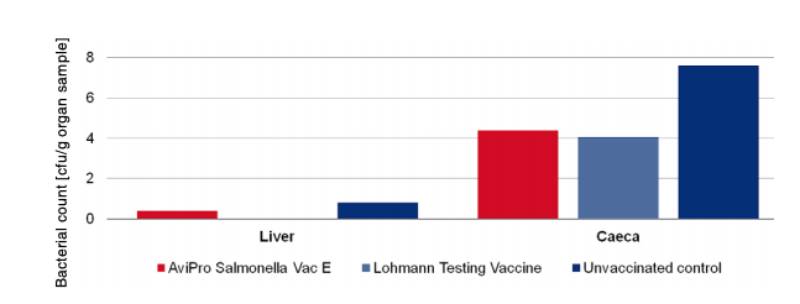
Figure 4: Persistence of the challenge strain Salmonella Typhimurium K284/93 Nalres in
internal organs 7 days post infection in week 62
In order to determine the protection against infection with Salmonella Typhimurium 10 birds were
challenged with 3 x 109 cfu of Salmonella Typhimurium K284/93 Nalres per bird and compared with birds
from the unvaccinated, challenged control group. Seven days post challenge infection the liver and
caeca of the vaccinated birds contained significantly less of the challenge strain than the unvaccinated
control birds (Figure 4).
In order to determine the protection against infection with Salmonella Typhimurium 10 birds were
challenged with 3 x 109 cfu of Salmonella Typhimurium K284/93 Nalres per bird and compared with birds
from the unvaccinated, challenged control group. Seven days post challenge infection the liver and
caeca of the vaccinated birds contained significantly less of the challenge strain than the unvaccinated
control birds (Figure 4).
Diagnostics
According to the European regulations (EC No. 213/2009 and EC No. 1168/2006) samples taken
from layer and breeder flocks are supposed to be tested for the presence of Salmonella by following
EN ISO 6579:2002. This method includes the use of modified semi-solid Rappaport-Vassiladis medium
(MSRV) as selective growth medium. Since growth of both Salmonella strains of the Lohmann testing
vaccine is rather poor on this medium, this method cannot be recommended for the determination of
the presence of the vaccine strains. Both strains can be detected as described previously in the
literature (Schröder et al., 2004). In addition the AviPro® Plate, a susceptibility microdilution test,
allows for a standardized test to detect and differentiate between the vaccine strain and Salmonella
field strains.
Conclusion
The Lohmann testing vaccine was proven to be safe and efficacious after challenge with high doses
of Salmonella Enteritidis as well as Salmonella Typhimurium, the most common Salmonella serovars
in poultry. Therefore, this new vaccine represents a new potent tool in the prevention of Salmonella
infections in poultry flocks and contributes highly towards establishing consumer confidence in safe
poultry products. The novel and unique combination of two live Salmonella strains in one vaccine
add to the development of user-friendly products in Salmonella prevention at farm level. In general,
vaccination alone cannot keep flocks free of Salmonella. Only a combination of high standards in
biosecurity and hygiene as well as proper vaccination with homologous vaccines can protect poultry
flocks against infection with Salmonella Enteritidis and Salmonella Typhimurium.
animal j; and eijk is the random residual effect. In the case of the cross-line data for laying rate, only
a cage average could be measured and only the sire was known. Therefore, in the model the cage
mean was used as vector of observations, and only one hen out of the half-sib family cage was used
for the animal effect.
Theoretically, the residual variance estimated from pooled observations should be about n times the
estimate based on individual observations (Biscarini et al., 2008). According to this author, the heritability
for pooled data should be recalculated as: h2 = σa
2 / (σa
2 + n σe
2); where ‘n’ is the number of hens/cage
(4 in this case). This is in line with Simianer and Gjerde (1991) who explained that estimation based
on group means contains less information on the group variation, which essentially contributes to the
estimation of residual variance components.
Results and discussion
Table 1 shows the means for all traits included in the analysis. Most people would expect higher
performance in single crosses than in the pure-breds due to heterosis, but as shown in the table this
is not always the case. It should be borne in mind that the pure-lines were kept under optimal biosecurity and management conditions, whereas the cross-breds were kept under less ideal commercial
environment in multiple-bird cages.
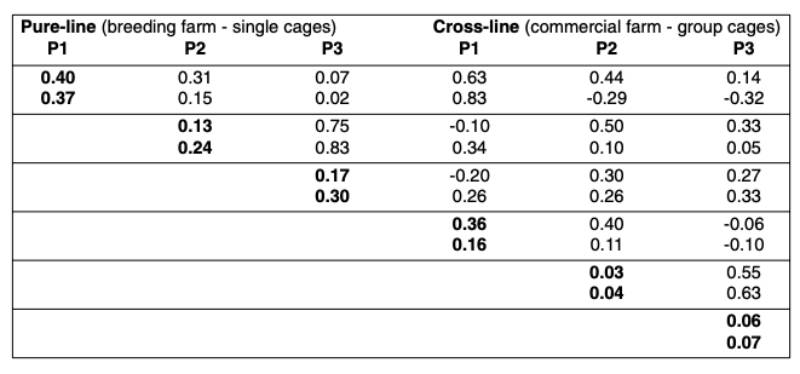
Table 2 shows the heritabilities and genetic correlations. Early egg production (P1) had the highest
heritability, which is explained by variation in age at sexual maturity (Preisinger and Savas, 1997).
The heritability was lowest during peak production and increased again towards the end of lay,
confirming estimates from the literature (e.g. Anang et al., 2000). No or a slightly negative correlation
was found between the production at sexual maturity and at the end of lay. As expected, the genetic
correlation was high between peak production and persistency.
The heritabilities were consistently higher based on full pedigree pure-line records from single cages
than based on the cross-line data from commercial multiple bird cages. The genetic correlations
between pure-bred and cross-bred performances were moderate to high at sexual maturity (rg = 0.63
and 0.83), whereas the correlations decreased to moderate to low levels in the other two stages of
production (rg between 0.1 and 0.5). The heritabilities obtained in this study were similar to those
published by Nurgiartiningsih et al. (2004).
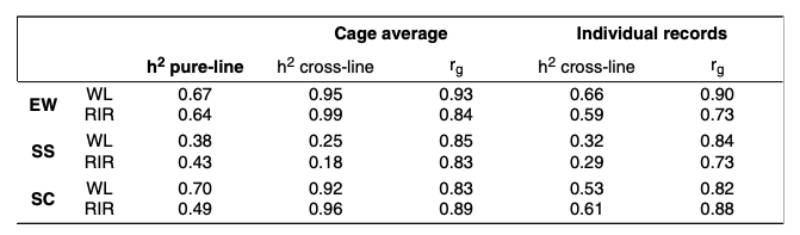
Estimates of genetic parameters for egg quality traits are summarized in table 3. For the cross-breds,
the individual egg quality measurements were used. Additionally, cage averages were assigned to
one of the hens in the cage and used for the analysis to underline the problems of using these traits
as a cage average. The heritability estimates based on cage averages are obviously too high and
reflect the reduced residual variance when using cage averages. Using the single egg records, the heritability estimates are slightly lower than in the pure-breds, which would be expected under “suboptimal” conditions. It should also be noted that only one egg per hen was evaluated for group cages,
whereas several eggs per hen were evaluated for the pure-lines in single cages.
Table 2: Genetic Parameters for egg production traits in pure-line and cross-line (cross-line
data as cage average, WL in the first row and RIR in the second)
Estimates of genetic parameters for egg quality traits are summarized in table 3. For the cross-breds,
the individual egg quality measurements was used. Additionally, cage averages were assigned to
one of the hens in the cage and used for the analysis to underline the problem of using these traits as
cage averages. The heritability estimates based on cage averages are obviously too high and reflect
the reduced residual variance when using cage averages. Using the single egg records, the heritability estimates are slightly lower than in the pure-breds, which would be expected under “suboptimal” conditions. It should also be noted that only one egg per hen was evaluated for group cages,
whereas several eggs per hen were evaluated for the pure-lines in single cages.
Table 3: Genetic Parameters for egg quality traits in pure-lines and cross-lines, with crossbred data as cage average and as individual records
Wei et al. (1995) concluded that combined cross-bred and pure-bred selection should be better than
selection based only on pure-line performance when improvement of cross-bred performance is the
goal. This strategy is generally more efficient when genetic correlations are below 0.8, which is the case
for laying rate. However, it is essential to find suitable farms with reliable data recording and flexible
housing schedules to match well-designed tests to augment the basis for selection focused on the
needs of the egg industry. If the residual variance is high due to uncontrolled factors which are not
representative for other farms, the heritability will be low and the gain from using this information
questionable.
Conclusions
The results of this study indicate that the genetic correlation between pure-line and cross-line egg
production was moderate, whereas the genetic correlations for all egg quality traits were high. This
confirms the working hypothesis that egg production data of cross-line relatives collected under
commercial conditions can contribute significantly to genetic improvement, while selection for egg
quality traits can be limited to pure-line records. The loss of statistical information on individual variation
due to working with cage mean is inevitable, but group cage data of cross-lines can help to reduce the
effect of genotype-environment interaction while taking heterosis effects into account. The parameter
estimates found in this study confirm that combined pure-line and cross-line selection is an efficient
approach to improve total genetic merit under field conditions.
Zusammenfassung
Salmonellen sind eine der Hauptursachen von Lebensmittel-bedingten Erkrankungen beim Menschen.
Noch immer sind kontaminierte Eier die Ursache für eine Vielzahl von Salmonellosen. Die Impfung von
Geflügelbeständen gegen Salmonella Enteritidis und Salmonella Typhimurium ist ein wichtiger
Bestandteil der Hygiene- und Biosicherheitsmaßnahmen, um eine Infektion in den Beständen zu
vermeiden. Der im Rahmen dieser Untersuchungen getestete homologe Kombinationsimpfstoff war
absolut unbedenklich und wirksam nach einer Belastungsinfektion mit virulenten Stämmen beider
Serovare.
Carrique-Mas, J. J. and R. H. Davies (2008): Salmonella Enteritidis in commercial layer flocks in Europe: Legislative
background, on-farm sampling and main challenges. Braz. J. Poult. Sci. 10:1-9.
Chacana, P. A. and H. R. Terzolo (2006): Protection Conferred by a Live Salmonella Enteritidis Vaccine Against Fowl
Typhoid in Laying Hens. Avian Diseases 50:280–283.
EFSA (2007): Report of the task force on zoonoses data collection on the analysis of the baseline study on the prevalence
of Salmonella in holdings of laying flocks of Gallus gallus. EFSA J. 97:1-84.
EFSA (2009): The Community Summary Report on trends and sources of zoonoses and zoonotic agents in the European
Union in 2007. EFSA J. 223:1-320.
Delmas, G., A. Gallay, E. Espié, S. Haeghebaert, N. Pihier, F. X. Weill, H. De Valk, V. Vaillant, and J. C. Désenclos. (2006):
Food-borne disease outbreaks in France between 1996 and 2005. B. E. H. 51/52:418-422.
Korsgaard H., M. Madsen, N. C. Feld, J. Mygind, and T. Hald. (2009): The effects, costs and benefits of Salmonella control
in the Danish table-egg sector. Epidemiol. Infect. 137:828-836.
Linde K., I. Hahn, and E. Vielitz (1996): Entwicklung von optimal an das Huhn attenuierten Salmonella-Lebendimpfstoffen.
Tierärztl. Umschau 51:23-31.
Majowicz, S. E., J. Musto, E. Scallan, F. J. Angulo, M. Kirk, S. J. O`Brien, T. F. Jones, A. Fazil, and R. M. Hoekstra. (2010):
The global burden of nontyphoidal Salmonella gastroenteritidis. Clin. Infect. Dis. 50:882-889.
Martin G., I. Hänel, R. Helmuth, A. Schroeter, W. Erler, and H. Meyer (1996): Investigations about the immunisation with
potential Salmonella Enteritidis mutants – 1. production and characterization in vitro. Berl. Münch. Tierärztl. Wschr.
109:325-329.
Newell, D. G., M. Koopmans, L. Verhoef, E. Duizer, A. Aidara-Kane, H. Sprong, M. Opsteegh, M. Langelaar, J. Threfall, F.
Scheutz, J. V. van der Giessen, and H. Kruse. (2010): Food-borne diseases – The challenges of 20 years ago still persist
while new ones continue to emerge. Int. J. Food Microbiol. 139(Suppl. 1): 3-15.
Schröder I., M. Iburg, and D. E. Rebeski (2004): Anwendung von TAD Salmonella vac® T und TAD Salmonella vac® E in
der Geflügelpraxis unter besonderer Berücksichtigung der Impfstoffapplikation und –kontrolle. Lohmann Information
4/2004:1-4.
Springer S., J. Lehmann, Th. Lindner, J. Thielebeln, G. Alber, and H.-J. Selbitz (2000): A new live Salmonella Enteritidis
vaccine for chicken – experimental evidence of its safety and efficacy. Berl. Münch. Tierärztl. Wschr. 113:246-252.
Stevens M. P., T. J. Humphrey, and D. J. Maskell (2009): Molecular insights into farm animal and zoonotic Salmonella
infections. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364:2709-2723.
Temelli, S., S. Kahya, A. Eyigor, and K. T. Carli (2010): Incidence of Salmonella Enteritidis in chicken layer flocks in Turkey:
Results by real-time polymerase chain reaction and International Organization for Standardization culture methods. Poult.
Sci. 89:1406-1410.
WHO. (2006): Progress Report (2000-2005): Building capacity for laboratory-based foodborne disease surveillance and
outbreak detection and response/WHO Global Salm-Surv. http://www.who.int/gfn/links/GSSProgressReport2005.pdf
 Linkedin
Linkedin








