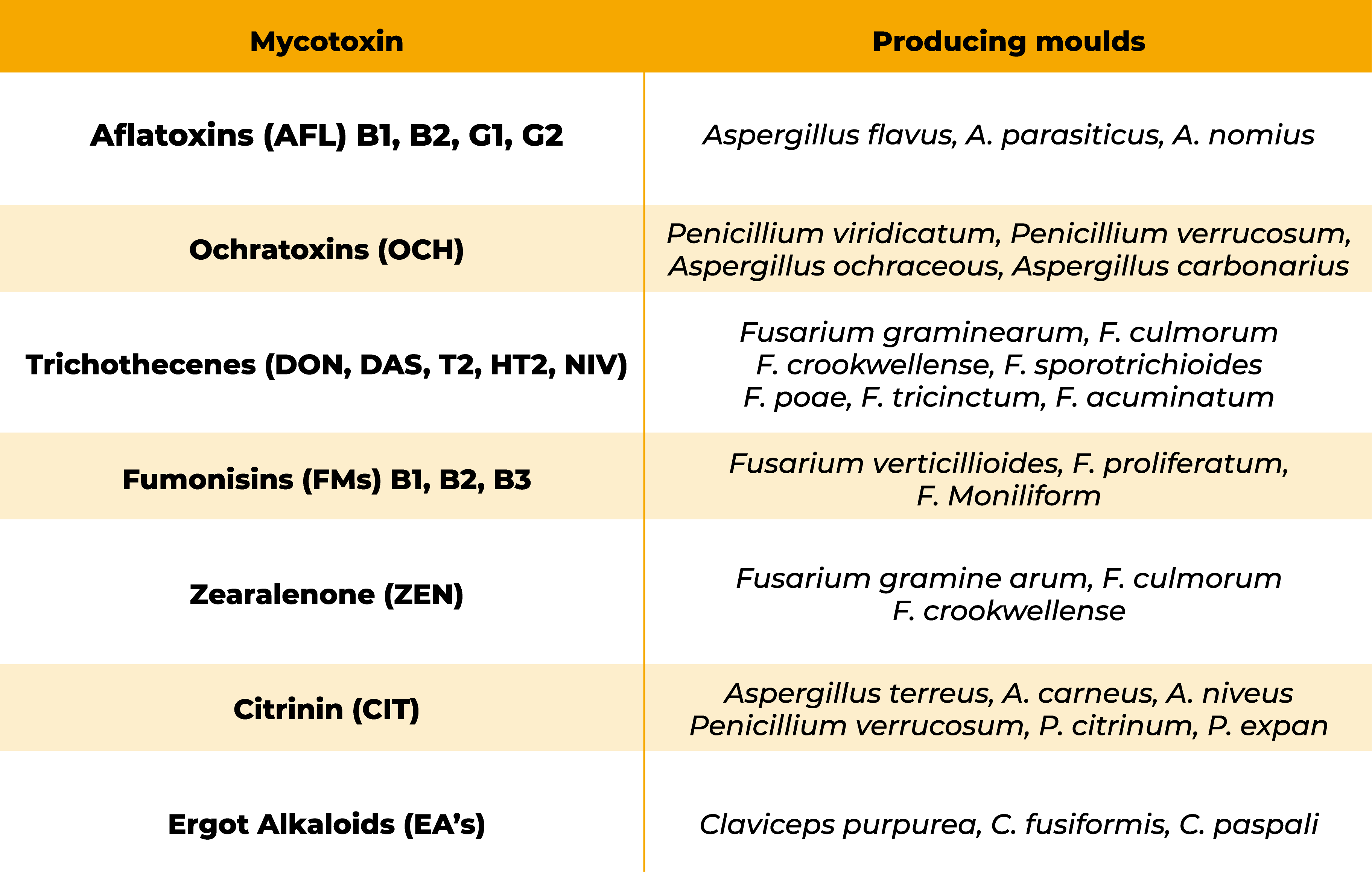
Mycotoxins are secondary metabolites produced by some species of Aspergillus, Penicillium, Fusarium, Claviceps and Alternaria genera that grow on different types of grains normally included in poultry diets.
-

These metabolites are normally produced as an adaptive response to environmental conditions (Pitt, 2000) during growth of crops, harvest, storage, transport, grain processing and even in animal feeders due to poor hygiene conditions.
Mycotoxin contamination of poultry feedstuff is a concern because they are potentially toxic for humans and animals and can lead to severe economic losses.
-

Currently, more than 500 secondary metabolites are reported, with aflatoxins, ochratoxins, trichothecenes, fumonisins, zearalenone, patulin, citrinin and ergot alkaloids representing the most relevant ones to humans and animals.
-

Their toxicity may vary considerably within a structural group and the effect may not always be due to the toxin itself but to its metabolites and possible synergistic effects in cases of multiple contamination which is now becoming more commonplace.
Description
Aflatoxins
Its major representative is aflatoxin B1 being the only group of mycotoxins clearly identified as carcinogenic for humans. Aflatoxins are toxic to the liver, immunosuppressant, hepatocarcinogenic, teratogenic, and mutagenic.
-
In laying hens, the effects of exposure to AF are dose-dependent leading to decreased laying rate and poor egg quality and has been reported to increase susceptibility to salmonellosis, candidiasis, and coccidiosis (Pier et al., 1980; Wyatt, 1991; Celik et al., 1996; Keçeci et al., 1998; Oliveira et al., 2002).
-
Transfer of aflatoxin B1 into eggs has been discovered in hens exposed to high concentration levels in feed. In laying hens, a maximum level of 20ppb is advisable.

Ochratoxins
The most representative is Ochratoxin A (OTA) which can be present in almost all cereals. It is mainly found in corn, barley, oats, rye, wheat and in oilseed products, particularly if they’re stored in an environment with a high moisture content.
-

OTA is mainly produced after harvest, thus being the predominant phase for contamination. Various studies have revealed toxic effects of OTA on the immune system and on the nervous system. It has also been reported to have dose dependant nephrotoxic effects.
-

In laying hens, it is associated with a drop in feed intake concomitant with an increase in water intake and reduction in egg production.
-

The recommended maximum level in laying hens’ diet should be below 250 ppb.
Trichothecenes
More than 100 compounds are known as Trichothecenes where the most prevalent are DON (deoxynivalenol, also known as vomitoxin), Nivalenol (NIV), 4,15-diacetoxyscirpenol (DAS), T-2 toxin and hydroxy-T-2 (HT-2).
-

Trichothecenes are produced by Fusarium sp., that can be present in most cereals during harvest and storage. Fusaric acid, that is often present in cereals, increases the toxicity of trichothecenes through a synergistic mechanism -Scudamore and Livesey, 1998.
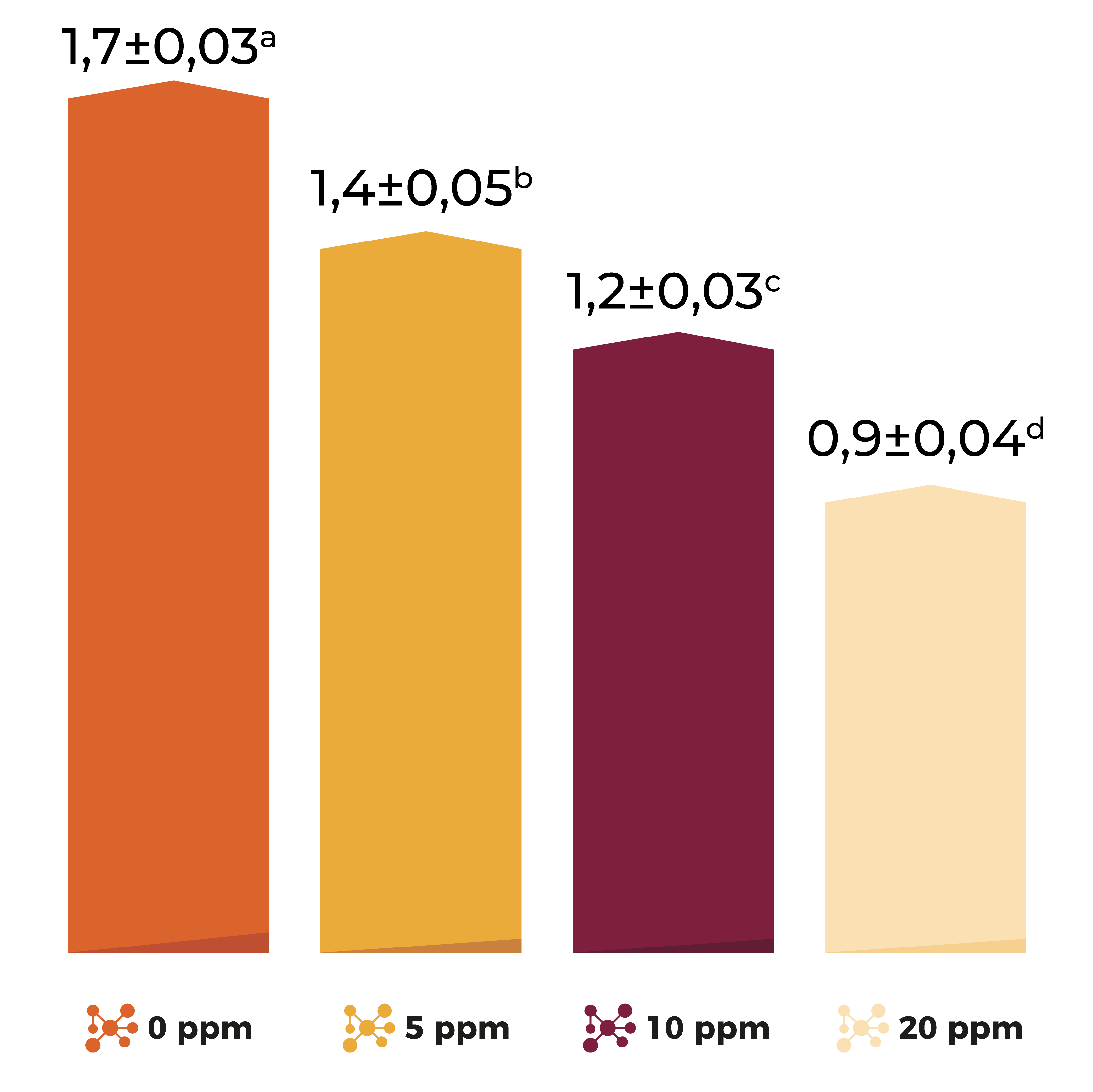
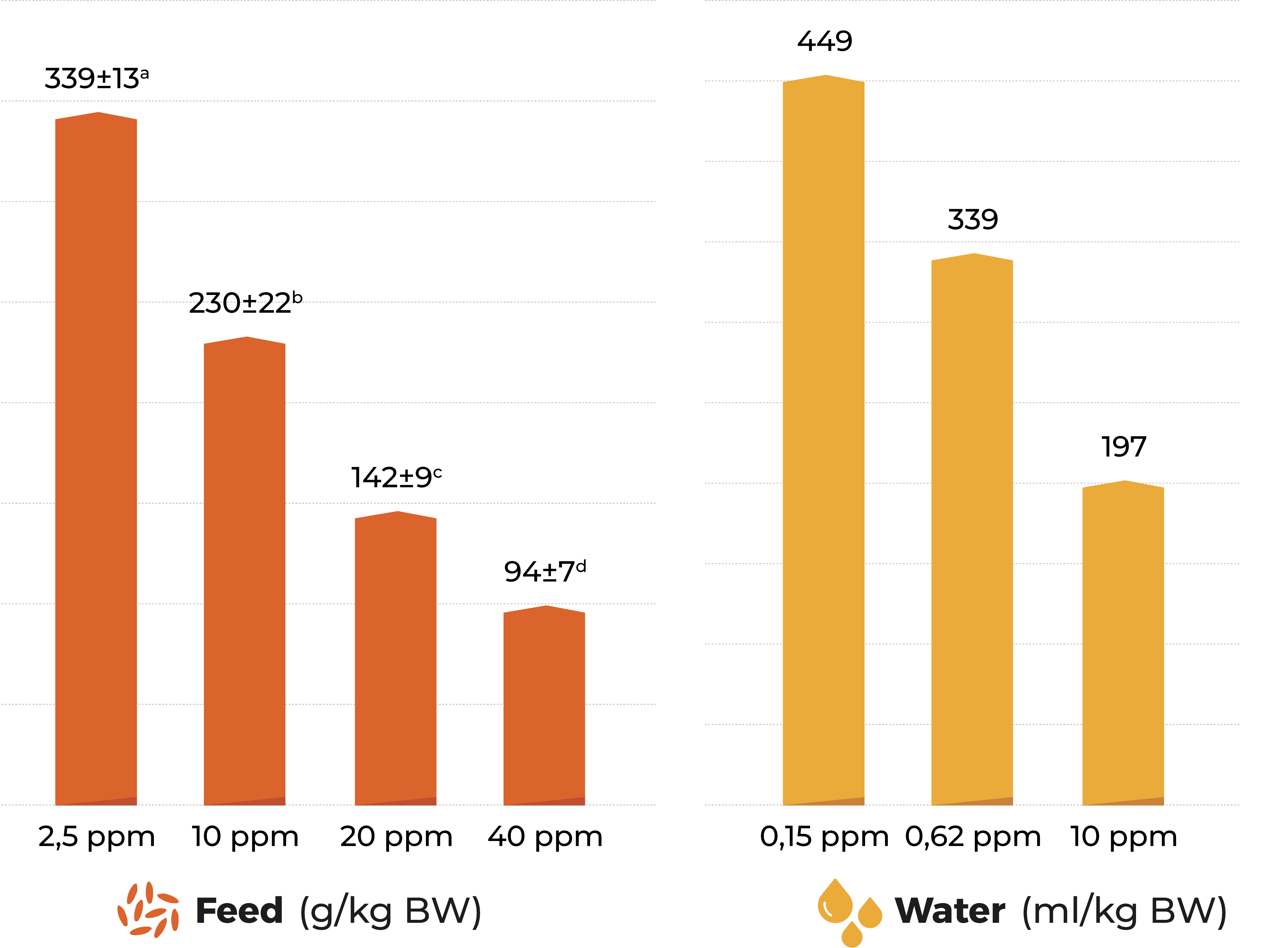
Main effects are an increase in feed consumption/refusal (Burditt, 1993), growth, reproduction, immune response, intestinal functions and can also affect cell membranes.
Effect of diacetoxyscirpenol (DAS) on weekly feed intake of broiler breeders. Brake et al., 2000
-

Maximum levels proposed in feed are 1000-1500ppb and 150ppm for T2 for a medium risk.
Fumonisins
Fumonisins (FB1, FB2, FB3) are mainly present in cereals, with F1 being the most prevalent in raw materials (corn and wheat) and feeds.
-

Oilseed and oilseed by-products can also be contaminated despite the fact they can be partially destroyed during oil extraction and further destroyed during processing.
-

Main effects observed are hepatotoxicity, immunomodulation, genotoxicity, and central nervous damage.
-
At cellular level sphingolipid synthesis can be disrupted (EU 2006/576). Effects on performance and mortality can be observed from 20ppm in feed.
Zearalenone
Zearalenone (ZEN) produced by several species of Fusarium being present mainly in corn and corn by-products but also in small amounts in sorghum, barley, wheat, and oats.
-
It’s normally found in areas with environmental warm weather.
-

ZEN and some of its metabolites are characterized by a high estrogenic activity which is the reason why it is associated with reproductive disorders.
-

In order to control feed contamination a maximum level of 500ppb is proposed.
Ergot alkaloids
Ergot alkaloids (EA) are mycotoxins produced by Claviceps purpurea mainly present in rye, triticale, and wheat.
-
The European Commission has established a maximum level of 1000mg/kg of rye ergot (C. purpurea) in feed whereas in the United States wheat containing 0.05% (500mg/kg) ergot is classified as “ergoty”.
-

Ergotism in poultry presents itself as a decrease in spontaneous activity, feed intake, growth, and egg production, often accompanied by diarrhoea.
Vesicles and ulcers develop on the beak, comb, wattles, and toes, and the combs and wattles can become atrophied and disfigured. Young poultry are more sensitive (Hoerr, 2019).

“Emerging” and “masked” mycotoxins
In addition to the “known” mycotoxins already regulated in some countries, AFB1, DON, ZEN, T-2, OTA, and FBs, during the last decade the development of liquid chromatography coupled with mass spectrometric methods (LC-MS/MS) allowed the screening of a wider number of mycotoxins in raw materials and poultry feeds called “emerging” mycotoxins
-

“Emerging” mycotoxins include Fusarium mycotoxins (fusaric acid, enniatin’s, beauvericin and moniliformin) and Alternaria mycotoxins (alternariol monomethyl ether, alternariol, and tenuazonic acid) that have not been regulated despite in vitro studies suggesting genotoxic, immunomodulating and reproductive toxic effects (Prosperini et al., 2013; Çelik et al., 2009,2010; Fleck et al., 2012, 2016).
Also, another group of mycotoxins that have recently emerged as an important co-contaminant in feedstuffs are plantderived conjugates called “masked” mycotoxins including deoxynivalenol3-glucoside, nivalenol-3-glucoside, zearalenol-14-glucoside, α-zearalenol14-glucoside, β-zearalenol-14-glucoside T-2-toxin-3-glucoside and HT-2-toxin-3-glucoside (Lolawole et al., 2020).
Mycotoxin’s evolution. Multi-contamination
We now see increasing occurrences of mycotoxins worldwide with a high variability in frequency and levels of contamination depending on the raw material considered, season and geographical region.
Due to this, we can surmise that environmental conditions have a key impact on mycotoxin molecular structure and contamination level before (field) and after the harvest (storage).
Within these environmental conditions, factors such as moisture content (water activity) of grains and feed, environmental temperature (there’s a wide array of temperature where mycotoxins may grow), CO2 availability, pH and presence of mould spores may have an impact on their development.
Obviously, climate change is affecting all these environmental conditions in a way that makes the contamination of raw materials included in poultry diets unpredictable, and therefore their toxic effects can’t be predicted just by summing up the individual toxicities.
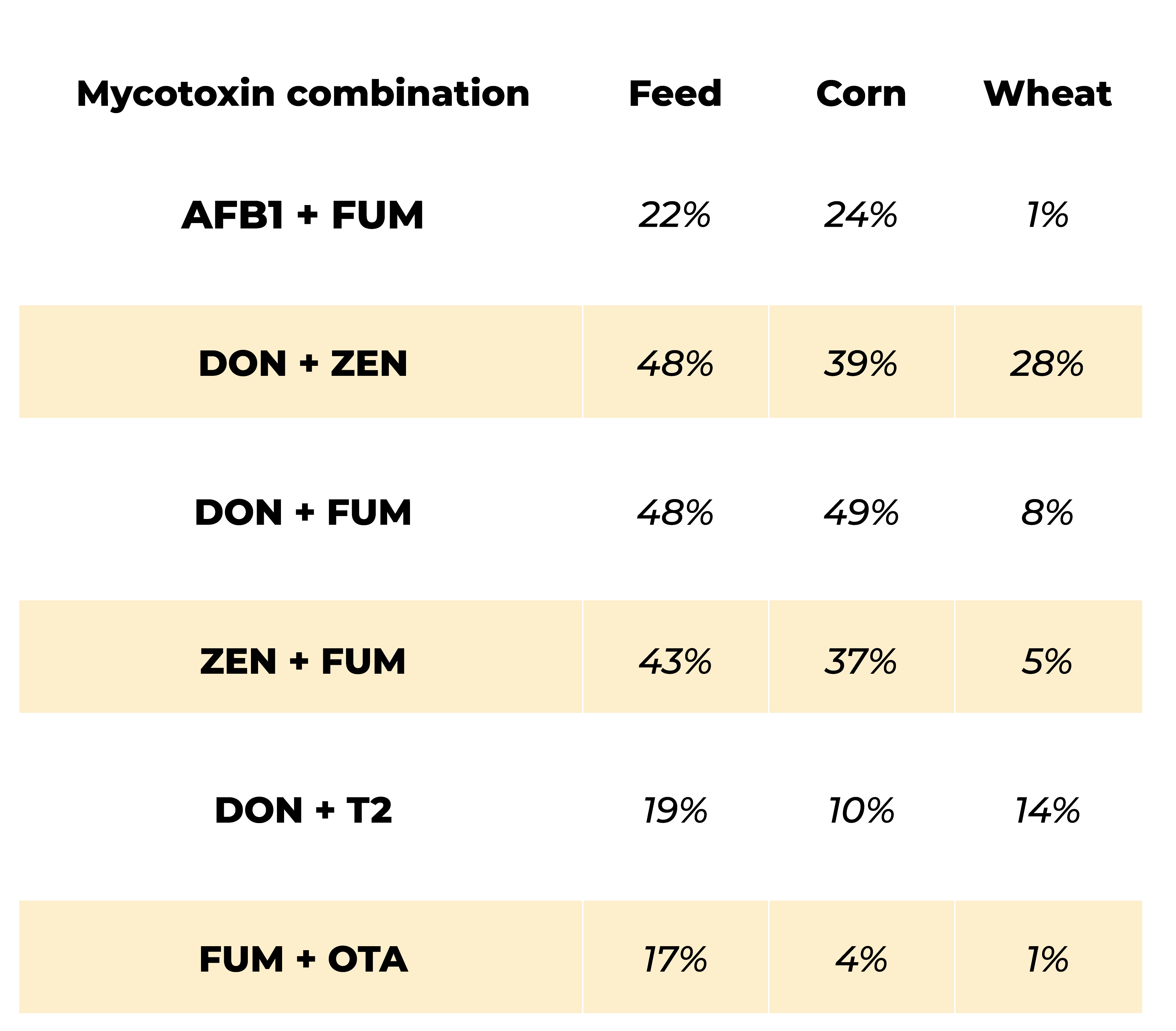
In addition to variability, feed generally contains multiple mycotoxins: in a survey from 2008 till 2017, 88% of samples of raw materials and feed were contaminated and 64% of them were contaminated with, at least, two mycotoxins DON, FUM and ZEN being the most prevalent (Gruber-Dorninger, 2019) whereas in another survey run by Mucio (2017) including 8452 feedstuff samples from 63 countries deoxynivalenol (DON) was present in 81% of samples, fumonisins (FUM) in 71%, 52% of the samples contained zearalenone (ZEN), 26% aflatoxin (AFLA), 19% T2 toxin, and 18% Ochratoxin (OT) where 76% of the samples contained more than one of them.
-

In addition, contamination in raw materials and feeds present an uneven distribution so, as the reader may understand, an adequate method for sampling is key when obtaining analytical results to help evaluate the risk and make informed decisions.
Mycotoxin’s toxicity
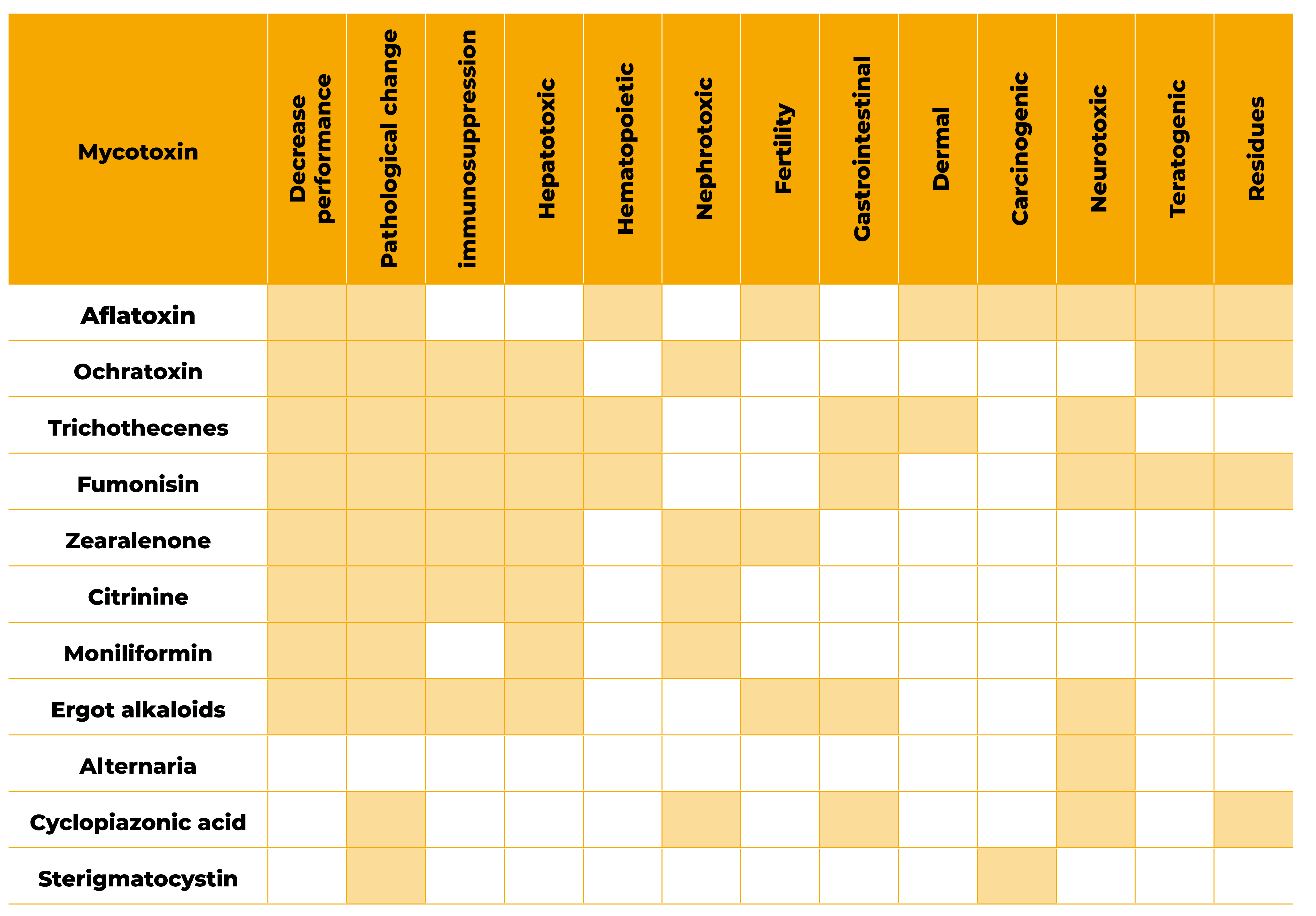
The manifestation of chronic or acute toxicoses depends on the dose, duration of exposure and rate of metabolism. Nowadays, mycotoxin levels found in feed are not high enough to provoke acute disease episodes, instead low levels may cause economical loss through subclinical changes in growth, production, and immunosuppression (Hamilton, 1982, Grenier and Oswald, 2011, Hoerr, 1991).
-

Frequently feed presents low levels of multi-contamination prolonged through time, causing chronic mycotoxicosis leading to a continuous stress on pullets and laying hens (i.e.: immunosuppression).
Visual effects may include reduced feed intake, feed refusal, poor feed conversion, diminished body weight gain, reduced hatchability and egg quality all leading to economic losses. It’s also important to bear in mind that when multicontamination occurs interactions among toxins happen, and their toxic effect may be additive, synergistic, or antagonistic, but still poorly understood.
Mohaghegh, A. et al (2016) reported reduced feed intake and feed efficiency as well as antibody titre against Newcastle disease and infectious bursal diseases in broilers fed a diet containing up to 0.4 mg/kg AFB1, 0.2 mg/kg OTA and 0.3 mg/kg DON.
Field and storage mycotoxins
Rearing companies and egg producers should assume that zero risk doesn’t exist and work on the basis that there’s no feed without mycotoxins present. Therefore, control of contamination represents the most effective approach to limit mycotoxin contamination in poultry diets.
-

Control starts with the crop. When possible, monitoring the crop during harvest and storing raw materials at cool and low moisture conditions is advisable to prevent mycotoxin production or, at least, keeping them under certain limits.
-

However, pre-harvest strategies are not completely effective, and fungal contamination of raw materials can lead to mycotoxin accumulation during storage (Loi et al., 2017). Then, during storage, maintaining and cleaning warehouses/bags and reducing moisture content of grains (max 10-13%) and oilseeds (max 7-8%) may help to control mycotoxin proliferation (Hell et al., 2008), however this might be difficult to perform specially in countries with a warm and/or humid climate.
-

Also, there’re a wide range of additives known to prevent both mycotoxigenic mould growth and mycotoxin formation (i.e.: organic acids).

Implementation of Good Agricultural Practices (crop rotation, pest-control, fungicides, etc..) can reduce the crop’s contamination in the field and also after harvest.
Also, a close collaboration/communication with suppliers (traders & distributors) is advisable.
Manufacturing. Feed production
Feed mills must control the quality of raw materials and feeds to guarantee both human and animal health.
-

Assuming that mycotoxins are present in raw materials, manufactures of pullets and laying hens feed must ensure that concentrations of these contaminants do not exceed the maximum allowed/recommended values.
-

Mycotoxins are generally heat stable; therefore, they’re not totally destroyed by the common methods applied (heat treatment, crumbling, pelleting and extrusion) as has been suggested.
-

Also, it is worth considering that pullets and laying hens feed is mostly provided in mash form (non-heat-treated most of the time).
-

Cereal grains normally represent the biggest inclusion rate of a hen’s diet which is the reason why they are our main focus, although cereal and oilseeds by-products can have higher levels of mycotoxins.
-

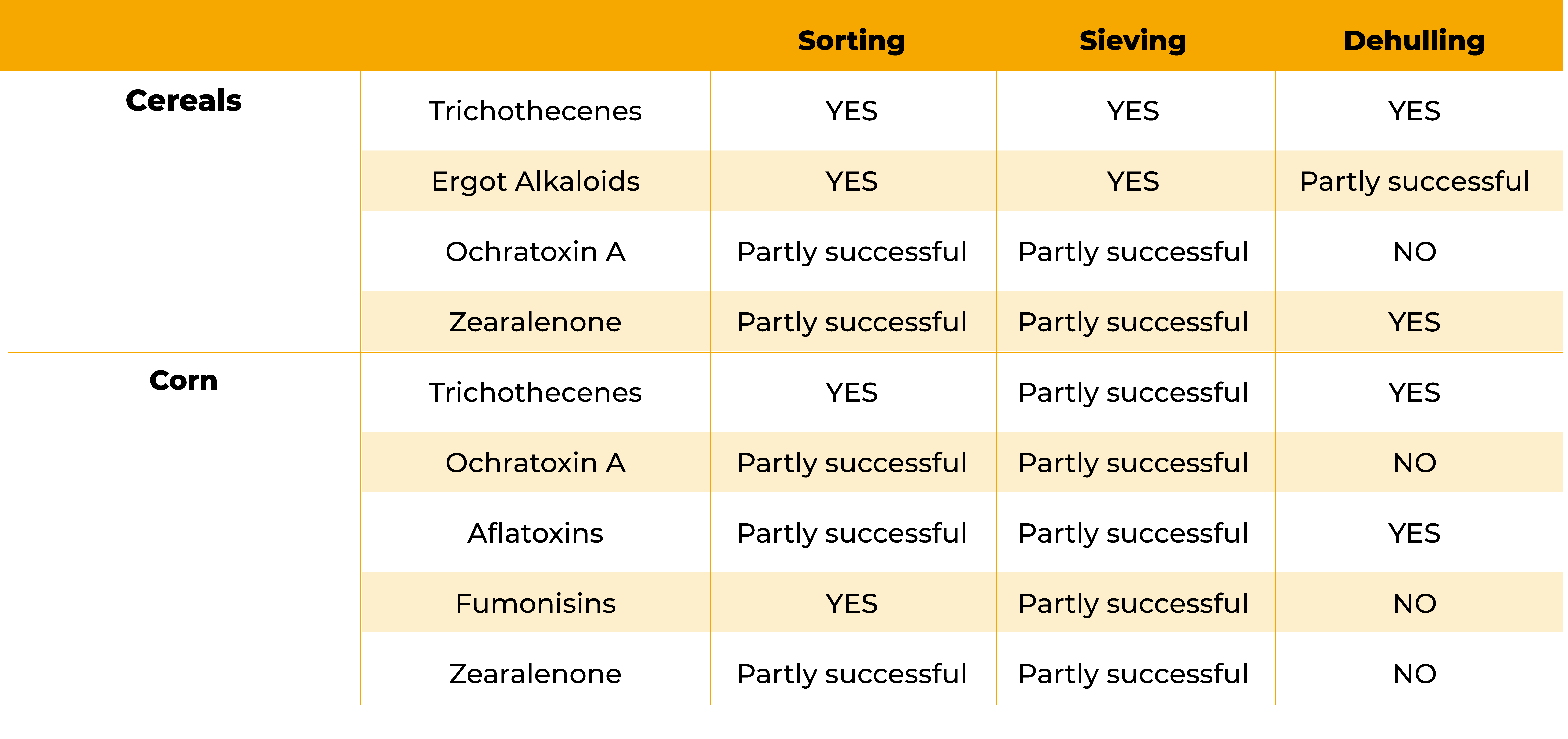
At the arrival point of the different raw materials, mycotoxin contamination can be reduced by cleaning (frequently accumulated in the grain – dust), sieving, removing broken grains and grains with moulds (Karlovsky et al., 2016).
-

Johansson (2006) pointed out how broken and damaged kernels usually contained most of the mycotoxin contamination.
-

In another study (Trenholm et al. 1991), found that by removing broken kernels and the smaller parts from maize, DON and ZEN contamination was reduced by around 70–80%.
At the feed factory, mycotoxins should be managed under HACCP (Hazard Analysis and Critical Point) as a preventive basis to control and monitor their presence and potential risk for human and animal health.
-

When manufacturing the feed, if contamination is known/suspected and local regulations allows it (not allowed anymore in Europe since 2003), the mycotoxin load can be reduced by “diluting” contaminated with non-contaminated raw materials therefore reducing their toxic effects.
-

However, feeding pullets with contaminated feed should be avoided at all costs since they’re most sensitive to contamination, especially during first weeks of age.

Feed additives
Many different methods (including chemical and biological) are used to mitigate/reduce/inactivate the effects of mycotoxins and their toxic metabolites present in raw materials and feeds
Mycotoxin binders
Mycotoxin binders are compounds with absorbent/binding/sequestrant properties. The efficiency varies considerably depending on the chemical characteristics of both the binder and the toxin plus the inclusion rate in the feed.
-

In addition, depending on the additive quality, some negative effects related to unspecific binding of essential nutrients (Huwig et al., 2001; Yiannikouris et al., 2006) have also been reported.
Working in a different extent they inhibit mycotoxin absorption in the gastrointestinal tract, therefore reducing the toxic effect. So far, no one single absorbent has proven to be effective against all types of mycotoxins.
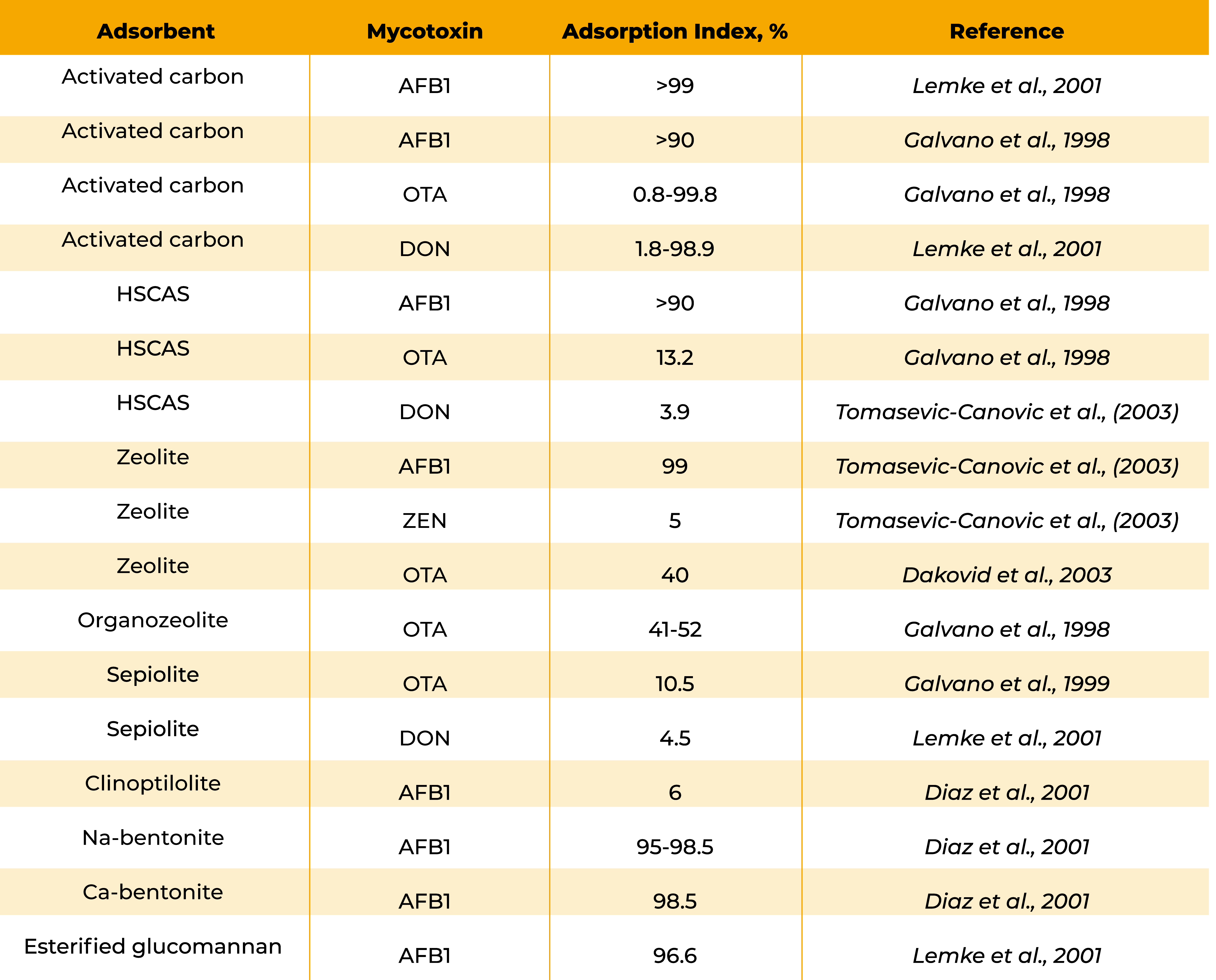
The composition of binding additives present in the market is variable for both inorganic and organic molecules. Within the organic ones, aluminosilicates, zeolites, bentonites, fuller’s earth, diatomaceous earth, activated charcoal, kaolin, clays (sepiolite- type), cholestyramine, smectite and montmorillonite are used as binders with all showing different characteristics.
-

As an example, some aluminosilicates inhibit the toxicity of AFTs but are not effective against trichothecenes, such as DAS or T-2 toxin (Kubena et al., 1993; Phillips, 1999) whereas zeolite has the potential to adsorb AFB1 and ZEA from feed (Peraica, 2002).
Table below shows some examples of In vitro adsorption of mycotoxins by different absorbents. (Kubak et al., 2006).
Some organic molecules (mainly indigestible carbohydrates) such as cellulose and complex cell walls like β-glucans, glucomannans, peptidoglycans, etc… have been shown to have a positive impact on reducing mycotoxins detrimental effects.
Cell walls are made of different constituents including polysaccharides, proteins, and lipids all with different absorption sites as well as numerous binding
mechanisms (Huwig, 2001).
The mode of action of the yeast wall mechanism occurs through adhesion to components of the cell wall (binding sites), rather than covalent binding or metabolic degradation, although the dead cells are still binding to toxins (Shetty et al., 2007).
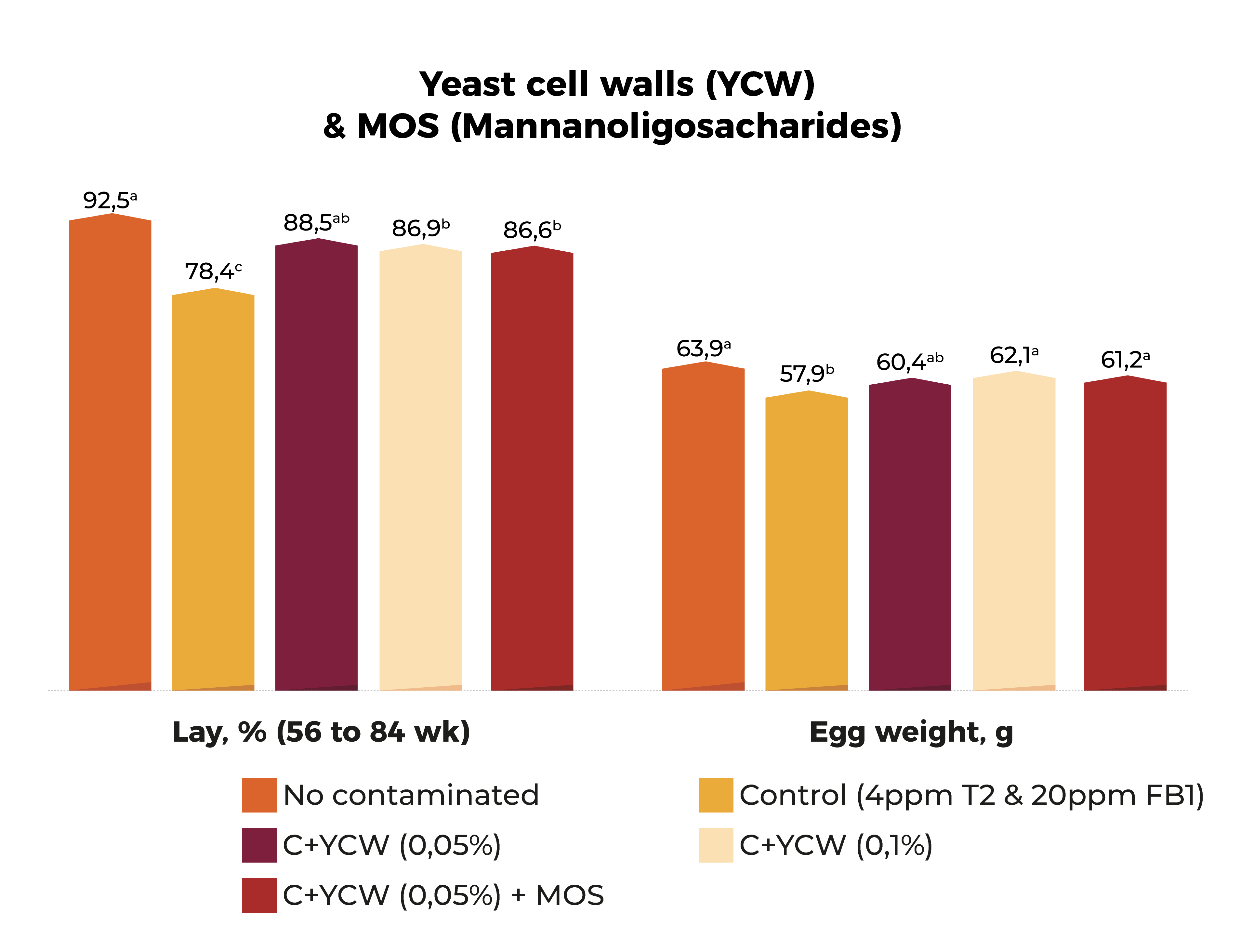
Jouany et al. (2005) has shown how the interaction between ZEN and β-glucans fractions of the yeast cell wall are “adsorptive-type” instead of a “binding-type”. Also, studies carried out during the last few years has shown how some microorganisms can inhibit mycotoxin absorption from feed.
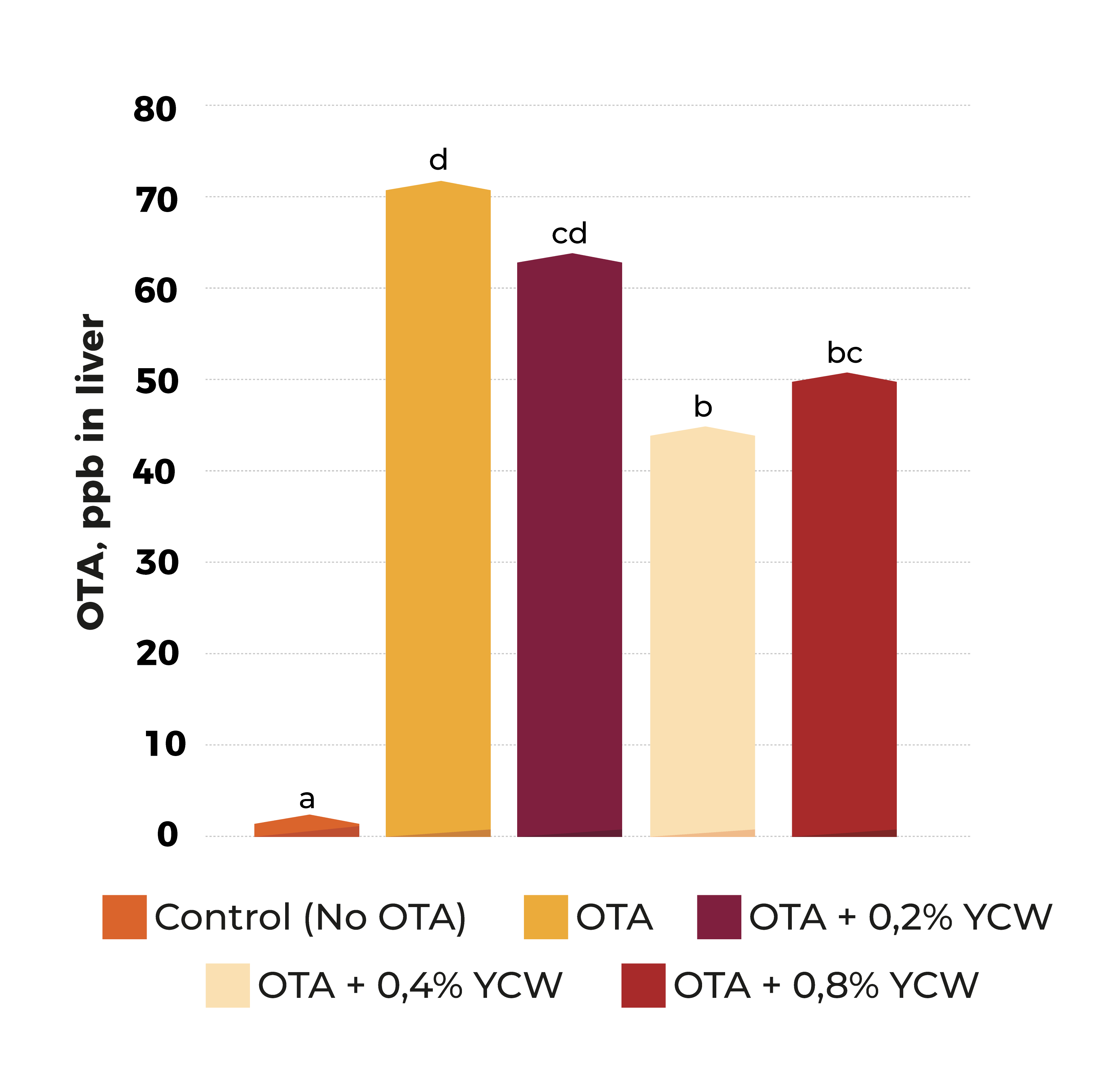
Biological transformation/détoxification. Mycotoxine modifier
Enzymes
During the last decades many studies took place searching for additives capable of detoxifying mycotoxins and transforming them into less toxic metabolites therefore reducing their effects (Schatzmayr et al., 2006).
-
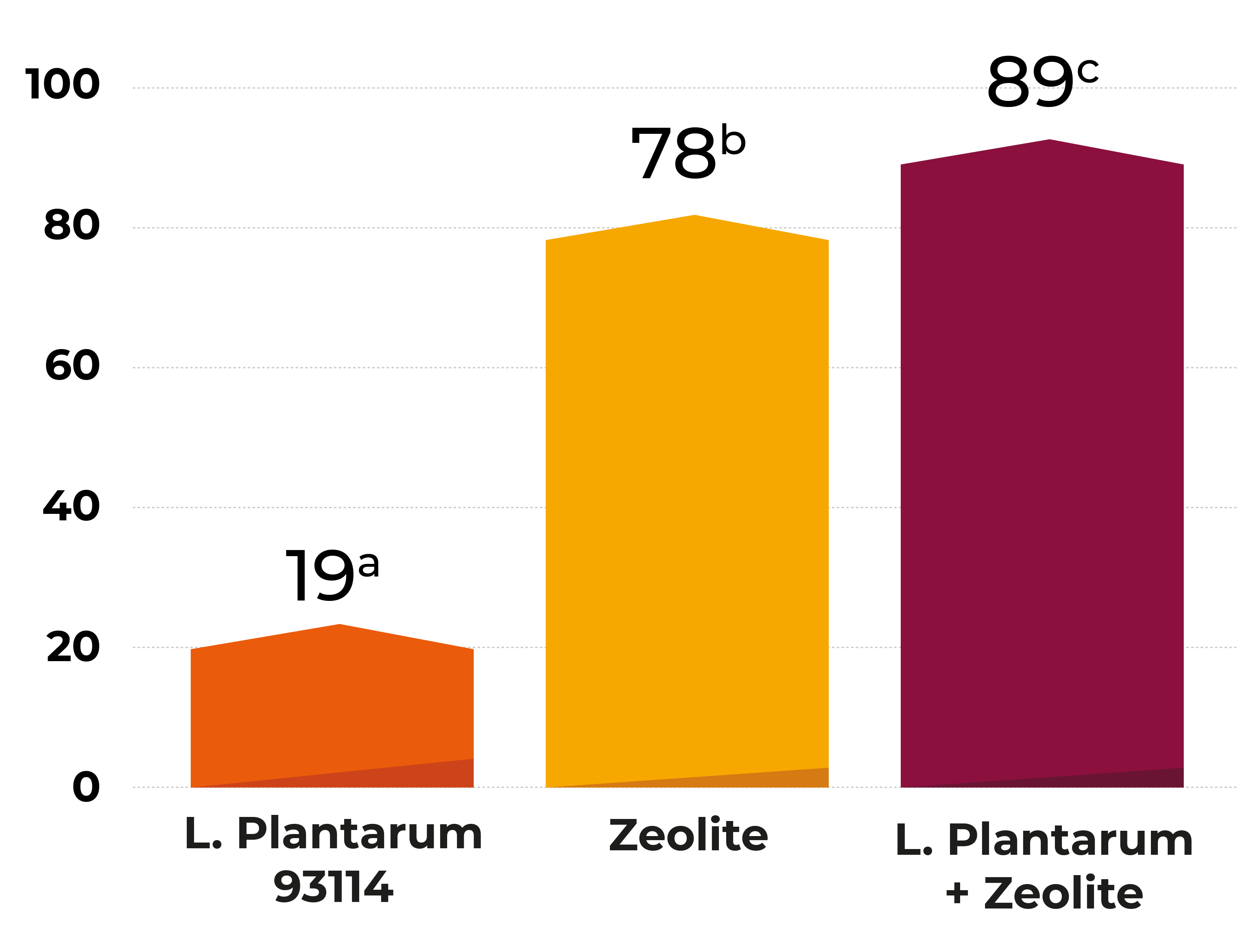
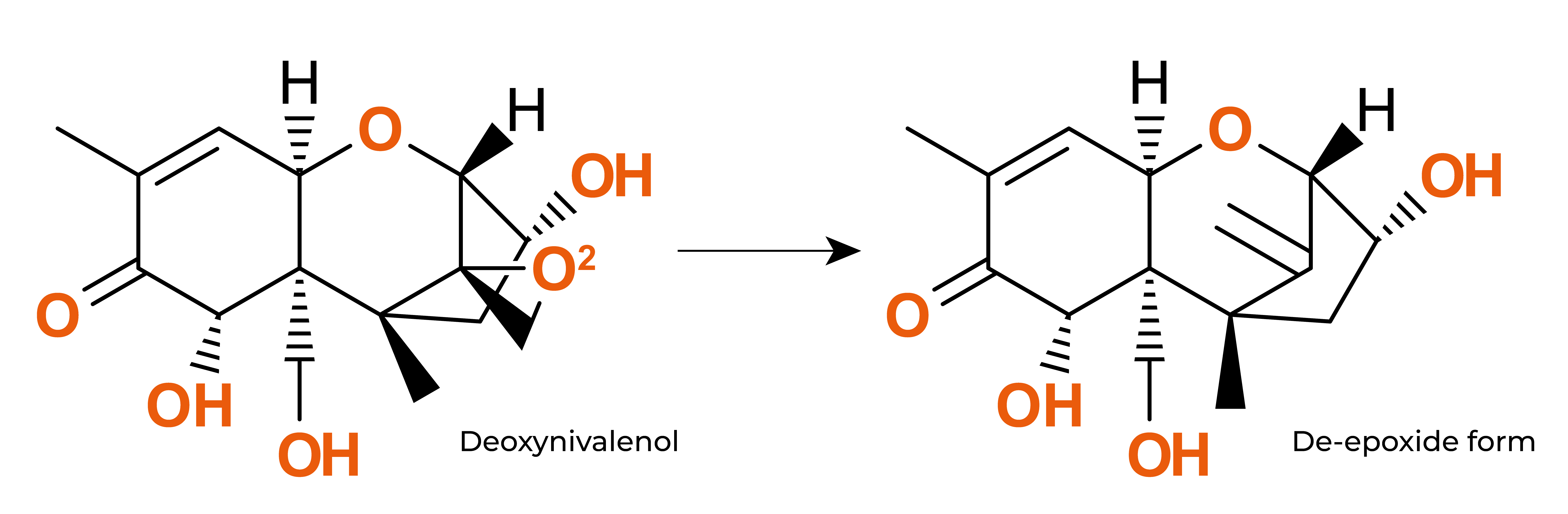
A wide range of microorganisms (being bacteria, moulds and yeasts the most common ones) and enzymes have already demonstrated their ability to transform them. Between them, microorganisms such as S. cerevisiae Rhizopus sp., Bacillus subtilis, Corynebacterium rubrum, Candida lipolytica, L. rhamnosus Aspergillus niger, Trichoderma viride, Mucor ambiguous, Neurospora spp., Armillariella tabescens, Gliocladium roseum or lactic acid bacteria have also shown their potential in transforming mycotoxins with varied efficiency to non- or less toxic products.
Summary
Mycotoxins are secondary metabolites produced by various toxigenic fungi belonging to the genera Aspergillus, Claviceps and Alternaria. Fungi may produce different mycotoxins and some mycotoxins can be produced by several fungi. Mycotoxins represent a risk for human and animal health.
Cereals and oilseeds and their respective by-products are commonly contaminated. Different companies are routinely screening levels of contamination worldwide.
“On top” of well-known mycotoxins there’s a number of less known mycotoxins called “emerging” and “masked” which are also presenting adverse effects.
Despite the fact it’s now feasible to detect large levels of mycotoxins, pullets and hens are more often exposed to multi-contamination at low levels where information about the extent of the effects on poultry species is scarce, but potentially can induce negative effects on the hen’s health, welfare, and productivity.
Instigating control practices by running analysis of raw materials and feeds is a must. It will help us to define our strategy. As always, prevention is better than cure.
The use of chemicals in combination with physical treatments increases the efficacy of mycotoxin degradation.










 Nutrition
Nutrition 