In broilers, layers and breeders, infections with avian pathogenic E. coli (APEC) are one of the most economically significant diseases in poultry.
Multi-factorial primary causes such as respiratory infections, immunosuppressive infections (e.g. Gumboro, Chicken Anemia) and environmental stress situations (e.g. extreme temperature changes, ammonia, dust) may lead to secondary E. coli infections. Even primary infections with certain very pathogenic Coli strains may result in septicemia, airsacculitis and egg peritonitis and are causes for increased mortality rates and reduced flock performance.
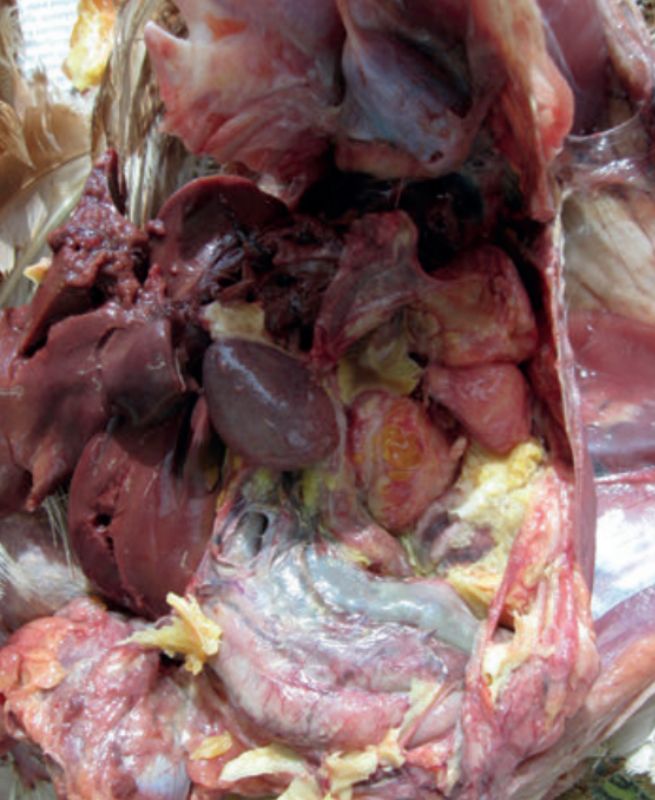
E. coli induced peritonitis
Besides indirect measures to control predisposing factors, antibiotic treatment of affected birds has also been common practice for many years. However, depending on local regulations, only a very limited number of antibiotics are licensed in most countries without any withdrawal period for the eggs. Furthermore, the general discussion about multi resistant bacteria, suchas ESBL’s as a potential risk for humans, limit the use of antibiotics to treat E. coli infections.
For many years now, autogenous inactivated vaccines against E. coli alone or in combination with other bacterial pathogens such as Pasteurella or Erysipelas have been used to control E. coli infections and to limit the economical impact of increased mortality, drops in egg production and reduced flock performance in future commercial layer or breeder flocks.
For some years now, in the United States and in other parts of the world, a live E. coli vaccine produced by Pfizer Animal Health, has been successfully applied. The vaccine Poulvac E. coli contains an aroA-Gen deleted E. coli O78 strain which is administered with a spray application as early as the first day of life. The vaccine strain lacks pathogenicity and induces cell-mediated immunity and local antibodies. In the field cross-protection against Coli serotypes has been demonstrated. The vaccine has now been approved by the European Medicines Agency (EMEA) in Europa as well.
The vaccine should be applied by coarse spray on day-old layers and breeders. The vaccine should neither be used within 6 weeks prior to the onset of egg production nor during the entire production period. The vaccine strain, which can be differentiated by laboratory techniques from E. coli field strains, could be re-isolated from organs of vaccinated chicks up to 6 days after vaccination and from the environment up to 5 weeks. As with all live bacterial vaccines, it is important to ensure that no antibiotics be used at least one week before and after vaccination as these will have a negative influence on the efficacy of the vaccination.
Hopefully, this new vaccine which will now be available in Europe, will demonstrate similar efficacy as reported from the U.S. and other countries. Nonetheless, as with all other vaccinations, the poultry farmers should not forget the basic requirements of management and bio-security in order to keep healthy birds.
Dr. Matthias Voss, Veterinary Laboratory
Multi-factorial primary causes such as respiratory infections, immunosuppressive infections (e.g. Gumboro, Chicken Anemia) and environmental stress situations (e.g. extreme temperature changes, ammonia, dust) may lead to secondary E. coli infections. Even primary infections with certain very pathogenic Coli strains may result in septicemia, airsacculitis and egg peritonitis and are causes for increased mortality rates and reduced flock performance.

E. coli induced peritonitis
Besides indirect measures to control predisposing factors, antibiotic treatment of affected birds has also been common practice for many years. However, depending on local regulations, only a very limited number of antibiotics are licensed in most countries without any withdrawal period for the eggs. Furthermore, the general discussion about multi resistant bacteria, suchas ESBL’s as a potential risk for humans, limit the use of antibiotics to treat E. coli infections.
For many years now, autogenous inactivated vaccines against E. coli alone or in combination with other bacterial pathogens such as Pasteurella or Erysipelas have been used to control E. coli infections and to limit the economical impact of increased mortality, drops in egg production and reduced flock performance in future commercial layer or breeder flocks.
These vaccines are unfortunately expensive to produce and need to be administered by injection. Furthermore, continuous work need to be done to follow the actual field strains, type them for virulence genes and adapt the vaccine strains for the next flock accordingly.
For some years now, in the United States and in other parts of the world, a live E. coli vaccine produced by Pfizer Animal Health, has been successfully applied. The vaccine Poulvac E. coli contains an aroA-Gen deleted E. coli O78 strain which is administered with a spray application as early as the first day of life. The vaccine strain lacks pathogenicity and induces cell-mediated immunity and local antibodies. In the field cross-protection against Coli serotypes has been demonstrated. The vaccine has now been approved by the European Medicines Agency (EMEA) in Europa as well.
The vaccine should be applied by coarse spray on day-old layers and breeders. The vaccine should neither be used within 6 weeks prior to the onset of egg production nor during the entire production period. The vaccine strain, which can be differentiated by laboratory techniques from E. coli field strains, could be re-isolated from organs of vaccinated chicks up to 6 days after vaccination and from the environment up to 5 weeks. As with all live bacterial vaccines, it is important to ensure that no antibiotics be used at least one week before and after vaccination as these will have a negative influence on the efficacy of the vaccination.
Hopefully, this new vaccine which will now be available in Europe, will demonstrate similar efficacy as reported from the U.S. and other countries. Nonetheless, as with all other vaccinations, the poultry farmers should not forget the basic requirements of management and bio-security in order to keep healthy birds.
Dr. Matthias Voss, Veterinary Laboratory