Abstract
The thermoregulatory system plays a central role in various body functions of the organism. It not only keeps the body temperature on an optimum level but also interacts with feed intake, growth rate, immune and stress responses. Perinatal temperature training (PTT) with short-term warm loads (+ 1°C, maximum 2 hrs/day) from day 18 until hatching, has shown long lasting effects on different traits. PTT decreased oxygen consumption during final embryonic development, improved hatching performance, changed the secondary sex ratio in favor to male chickens, and improved performance during the post-hatching growing phase by better weight gain and feed conversion. Improvement of hatching results and post-hatching performance was found in small and large scale experiments. Further, long-lasting changes in physiological parameters (HLR, metabolic hormones T3/T4) indicate better stress response through PTT. Similar results have been found in ducks with short-term cold training. It is assumed that PTT during the last days of hatching reduces the metabolic rate. Hence, the birds have more energy available for performance as well as for adaptation, immune and stress responses during environmental challenges. In conclusion, PTT improves robustness and production efficiency. It is a practicable epigenetic tool, in chickens and ducks which enables realization of the genetic determined performance. Through better adaptation to changing environmental conditions PTT also contributes to the bird`s welfare.
Keywords
Incubation, broilers, ducks, perinatal temperature, adaptation, performance
Introduction
According to the Agricultural Outlook 2020 poultry meat will be the largest meat sector in the world (OECD/FAO, 2014). Continuous genetic improvement in poultry breeds led to a fast development in performance of commercial poultry lines. Broilers in particular are characterized by high growth and metabolic rate already during embryonic development (Janke et al.; 2004, Druyan, 2010; Buzala et al., 2015). High performance and increased metabolic heat production result in relative low capacity of heat dissipation and increased susceptibility to stress under changing environmental conditions (Havenstein et al., 2003; Schmidt et al., 2009; Kalmar et al., 2013; Loyau, 2015). The higher risk of high yielding poultry lines for behavioural, physiological and immunological problems is an important animal welfare issue. Improvement of robustness alongside with high production efficiency has top priority. Recent studies using short-term temperature stimulation during critical developmental periods in the hatching phase (perinatal temperature training, PTT) have shown positive effects on robustness, health and performance in fast growing chickens and ducks.
In the following we review the physiological background of this method and present results of small-scale and large-scale experiments.
Physiological background of PTT
How does temperature training improve robustness and production efficiency?
Temperature regulation plays a central role in the hierarchy of regulatory systems in homoeothermic (warm-blooded) animals. The goal of temperature regulation is the maintenance of a constant body core temperature under most conditions. It is the prerequisite for optimum development and performance. The thermoregulatory system integrates different body functions. The close relationship between thermoregulation and other homeostatic physiological functions occur already on cellular level in the regulatory center of the brain, the hypothalamus. Hypothalamic neurons receive and process temperature, metabolic, osmotic and hormonal signals (Boulant and Silva, 1989; Hori, 1991). Hence, adaptation to temperature may also influence feed intake, body weight development, metabolism, immune and stress response, and reproduction (DuRant et al., 2012; Horowitz, 2014; Nassar et al., 2014). Finally, PTT may improve robustness in poultry (Boerjan, 2010; Tzschentke and Tatge, 2012).
Why is the hatching phase optimum for application PTT?
During the last days before hatching the embryo is prepared to respond to environmental stimuli. Physiological mechanisms have evolved from open loop to closed feedback control systems (Tzschentke and Plagemann, 2006, Tzschentke, 2007; Tzschentke and Rumpf, 2011). Closed feedback systems are a prerequisite of efficient PTT. During the perinatal period environmental influences can have long-lasting and obviously transgenerational effects (Tzschentke and Plagemann, 2006, Tzschentke et al., 2014, 2015a). Improvement of temperature adaptation and growth in relation to perinatally experienced chronic temperature changes were already demonstrated in chickens about 30 years ago from Minne and Decuypere (1984) and Decuypere (1984). In our own research in Muscovy ducks we found long-lasting effects of changes in incubation temperature during final embryonic development on post-hatching sensitivity of hypothalamic neuronal mechanisms, which were related to improved temperature adaptation and stress response (Tzschentke and Basta, 2002; Janke and Tzschentke, 2010).
Why is heat production declined by short-term mild warm PTT?
Mild short-term warm stimulation during final embryonic development decreased oxygen consumption and heat production in layer- and meat-type chicken as well as in Muscovy duck embryos (Nichelmann et al., 1994; Janke et al., 2002; El-Sabry and Tzschentke, 2010). Birds are naturally incubated under conditions of ‘summit metabolism’ (Nichelmann et al., 1994; Tzschentke and Rumpf, 2011). Heat production decreases with increasing incubation from the natural incubation temperature of about 37.5°C towards thermoneutral conditions of about 39°C (Figure 1). Above thermoneutrality heat production increases again. This shows that the response of heat production to changes in the ambient (incubation) temperature of the embryo is similar to that observed in the post-hatching and adult state (Tzschentke and Nichelmann, 1999; Nichelmann and Tzschentke, 2002). During a mild increase in hatching temperature heat loss mechanisms (e.g. respiration, blood circulation) are effective to keep deep body temperature constant (Holland et al., 1998; Nichelmann and Tzschentke, 2002, 2003; Tzschentke 2007). This could be demonstrated in experiments where deep body temperature was measured in the colon of the embryo (Holland et al., 1998; Tzschentke 2007). We concluded that mild warm PTT decreased oxygen consumption and heat production in embryos of high yielding broilers (Figure 2). The daily PTT training during ‘critical period’ in the development of thermoregulation might programme the thermoregulatory system and homeostatic functions. The long-lasting result would be a lower basic metabolism, which enables better weight gain and feed conversion rate. Under these conditions there is energy available to cope with stress and immune challenges. Thus, PTT improves robustness and performance.
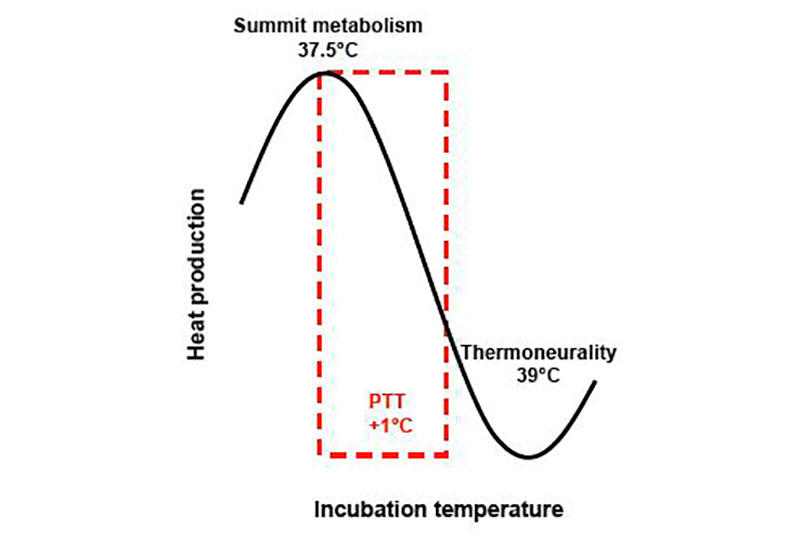
Figure 1: Schematic diagram of the relationship between oxygen consumption and incubation temperature of late term poultry embryos (PTT: perinatal short-term temperature training, temperature range)
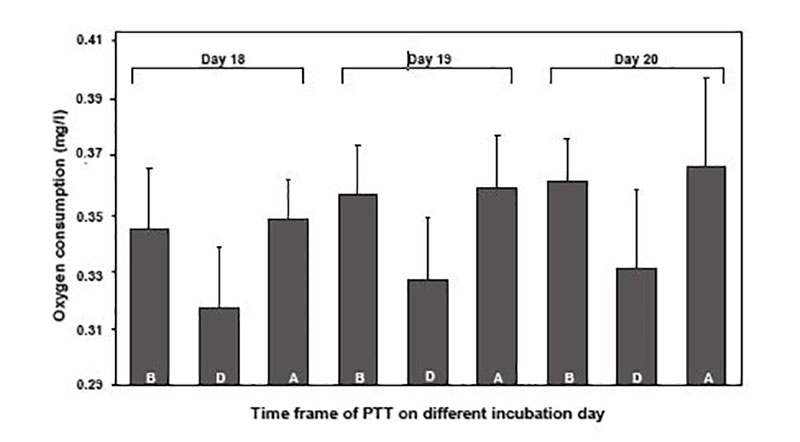
Figure 2: Influence of perinatal short-term temperature training (PTT, + 1°C for 2 hrs daily) on oxygen consumption of Cobb broiler embryos on incubation days 18, 19 and 20 (N=7). Columns represent mean values of oxygen consumption measured for 60 sec. before (B), during (D) and after (A) PTT (El Sabry, M.I., B. Tzschentke, 2010)
Studies on PTT in broilers in small and large scale tests
In small scale experiments (about 1000 eggs per treatment) with Ross 308 broilers (Tzschentke and Halle, 2009) we demonstrated for the first time that PTT (maximum + 1°C, 2 hrs/day) increased hatching rate and body weight of hatched chicks, and changed the secondary sex ratio (percentage of vital male and female chicks hatched in relation to total chicks hatched) (Figure 3). Short-term warm stimulation improved hatchability by more than 1.5% and was associated with a significantly higher proportion of hatched male chickens. In subsequent growth trials a better feed conversion mostly along with better body weight gain up to the slaughter age (35 days) were observed (Tzschentke and Halle, 2009). The positive effects were preferentially found in males (Table 1). Similar effects have been reported by Elmehdawi et al. (2015) in a large scale experiment (incubators for 42,240 eggs) using a stimulation of + 0.5°C for 2 hrs per day from 18th day of incubation until hatching. The proportion of vital hatched male chickens was significantly increased by 2.2 to 3.5% in response to PTT. Examination of the unhatched eggs confirmed the hypothesis that PTT improves the robustness already of the embryos, and especially in the males. The percentage of unhatched male embryos was significantly higher in the control group. Similar effects were found if PTT of +1°C was applied, even the increased percentage in male hatched chickens was not statistically significant higher (Elmehdawi et al., 2016). However, long-term effect of PTT on performance was only found after PTT of + 1°C (Elmehdawi et al., 2016). In this experiment feed conversion was improved by about -2% which was similar to results from the small scale experiment (Tzschentke and Halle, 2009).
First experiments on stress response showed that PTT treated chickens had a significantly lower heterophil to lymphocyte ratio (HLR) in comparison with the normally incubated control birds. During severe stress male broilers showed significantly higher thyroid hormone levels (T3/T4) in the blood, which demonstrate higher ability to mobilize energy for the stress response (Tzschentke et al., 2015b).
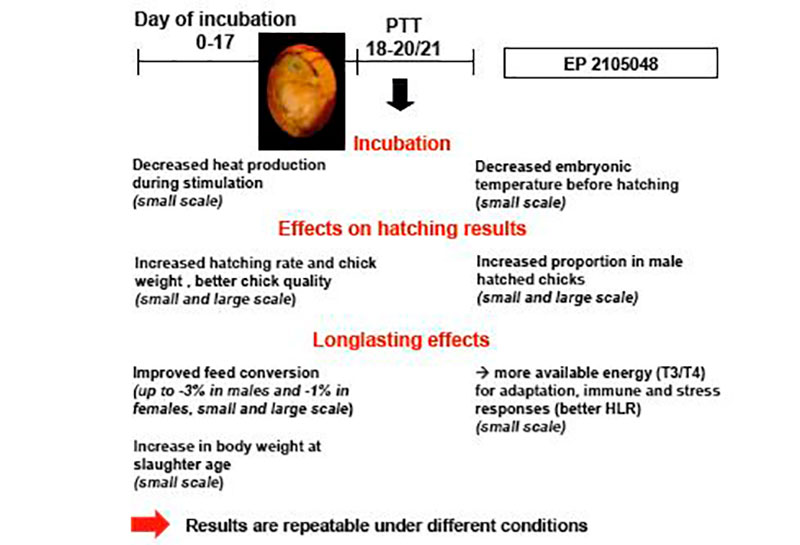
Figure 3: Summary of the results of perinatal short-term temperature training (PTT) on robustness and performance in broiler chickens from small and large scale experiments (results from large scale experiments, Elmehdawi et al., 2015, 2016; image ©Pasreform Hatchery Technologies)
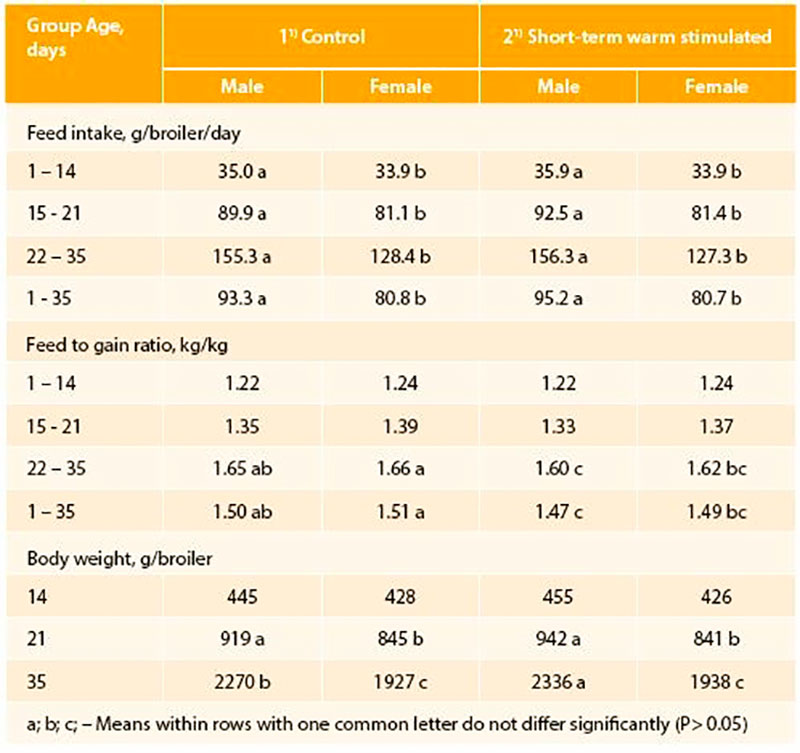
Table 1: Performance of normally incubated (Control) and perinatal temperature stimulated (4 days, 2 h per day, + 1°C) broiler chickens (n=120/group/sex; Tzschentke and Halle, 2009)
Perspectives of PTT application in other poultry species
In other poultry species as Pekin ducks short-term temperature training in the hatching phase was also tested. Whereas short-term mild warm stimulation as in the broiler chickens depressed hatching results in ducks, short-term cold stimulation (1°C below standard incubation temperature for 2 hrs per day) had long-lasting effects on the ducks performance (Halle et al., 2012). Short-term mild cold stimulation during the last days before hatching improved body weight gain and feed conversion during the growing period exclusively in male ducks. Further, at slaughter age male and female ducks from the short-term stimulated group reached statistically higher crude protein content in the breast meat compare to ducks of the control group (Table 2 and 3). Obviously, ducks need different temperature stimulation than chickens, because of their better cold tolerance and higher maturity of body functions as termoregulatory heat production already during final incubation (Sallagundala et al., 2006).
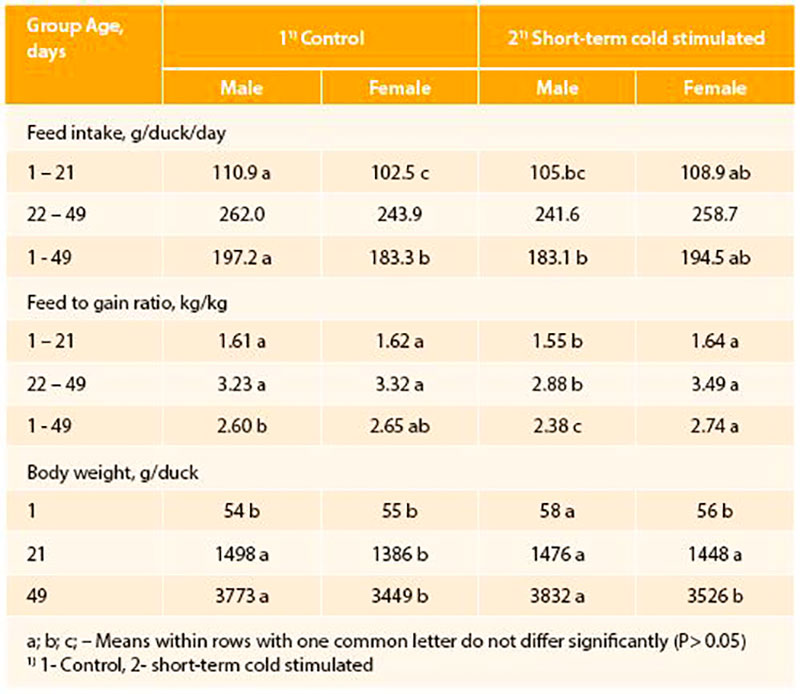
Table 2: Performance of normal incubated and perinatal temperature stimulated (6 days, 2 h per day; -10C) Pekin ducks (n=72/group/sex; Halle et al., 2012)
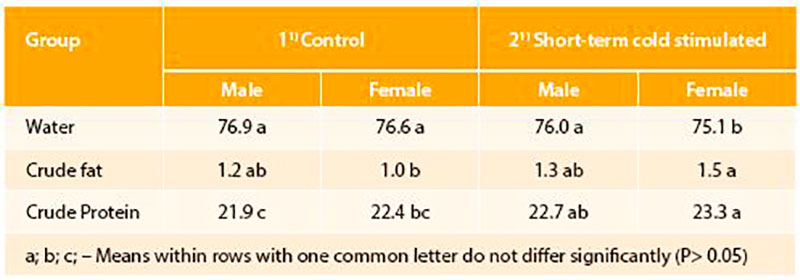
Table 3: Content (%) of water, protein and fat in the fresh matter of the breast meat of normally incubated and perinatally temperature stimulated (6 days, 2h per day; -10C) Pekin ducks (n=6/group/sex; Halle et al., 2012)
Conclusions
In conclusion, in broiler chickens robustness alongside with production efficiency can be improved by PTT as a practicable epigenetic tool. Advantages of an epigenetic method would be the relatively simple application of nature like changes in environmental factors during incubation as well as the short-term achievement of practice relevant results (already in the first generation). In chickens, short-term mild warm stimulation during a critical period of body temperature control development may program the basic metabolism on a lower level. This would enable better activation of environmental adaption processes, stress- and immune responses as well as better realization of the genetic determined performance potential. To improve the PTT protocols for other poultry species, more research on embryonic physiology as well as on its long-term effects is necessary.

References
Boerjan, M. (2010) Circadian incubation. International Hatchery Practice 24: 13-15.
Boulant, J., N. Silva (1989) Multisensory hypothalamic neurons may explain interactions among regulatory systems. Physiology 4: 245-248.
Buzala, M., B. Janicki, R. Czarnecki (2015) Consequences of different growth rates in broiler breeder and layer hens on embryogenesis, metabolism and metabolic rate: a review. Poult. Sci. 94: 728-733.
Decuypere, E. (1984) Incubation temperature in relation to postnatal performance in chickens. Arch. Exp. Vet. Med. 38: 439-440.
Dörner, G. (1974) Environment-dependent brain differentiation and fundamental processes of life. Acta Biol. Med. Germanica 33: 129-148.
Druyan, S. (2010) The effect of genetic line (broilers vs. layers) on embryonic development. Poult. Sci. 89: 1457-1467.
DuRant, S.E., W.A. Hopkins, D.M. Hawley, G.R. Hepp (2012) Incubation temperature affects multiple measures of immunocompetence in young wood ducks (Aix Sponsa). Biol. Lett. 8: 108–111.
Elmehdawi, A., M.A. Hall, P.A. Skewes, D.L. Wicker, D.V. Maurice, J. Smith, R. Benton (2015) Low-intensity short-duration thermal stimulation during the late phase of incubation alters secondary sex ratio in favour of males. Brit. Poult. Sci. 56: 381-388.
Elmehdawi, A., M.A. Hall, P.A. Skewes, D.L. Wicker, D.V. Maurice (2016) Influence of thermal stimulation during the late phase of incubation on hatching results and posthatch broiler performance under commercial conditions. Brit. Poult. Sci. http://dx.doi.org/10.1080/00071668.2016.1209737-
El Sabry, M.I., B. Tzschentke (2010) Influence of short-term warm stimulation during the last 4 days of incubation on body temperature and oxygen consumption in broiler and layer embryos. Avian Biol. Res. 3: 129-130.
EP 2105048 A1: A method for growing poultry, 20090930 (EN), Humboldt-Universität zu Berlin, inventors I. Halle, B. Tzschentke.
Halle, I., B. Tzschentke, M. Henning, P. Köhler (2012) Influence of temperature stimulation during the last 6 days of incubation on hatching results and later performance in Pekin ducks. Arch. Geflügelk. 76: 176-183.
Havenstein, G.B., P.R. Ferket, M.A. Qureshi (2003) Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82: 1500-1508.
Holland, S., J. Höchel, A. Burmeister, O. Janke, M. Nichelmann (1998) A method for measuring deep body temperature in avian embryos. J. Therm. Biol. 23: 123–129.
Hori, T. (1991) An update on thermosensitive neurons in the brain: From cellular biology to thermal and non-thermal homeostatic functions. Jap. J. Physiol. 41: 1-22.
Horowitz, M. (2014) Heat acclimation, epigenetics, and cytoprotection memory. Compr. Physiol. 4: 199-230.
Janke, O., B. Tzschentke (2010) Long-lasting effect of changes in incubation temperature on heat stress induced neuronal hypothalamic c-Fos expression in chickens. In: Tzschentke, B. (Ed.) Special Issue: Early development and epigenetic programming of body functions in birds. TOOJ 3: 150-155.
Janke, O., B. Tzschentke, M.L. Boerjan. (2004) Comparative investigations of heat production and body temperature in embryos of modern chicken breeds. Avian Poult. Biol. Rev. 15: 191-196.
Janke, O., B. Tzschentke, J. Höchel, M. Nichelmann (2002) Metabolic responses of chicken and Muscovy duck embryos to high incubation temperatures. Comp. Biochem. Physiol. A 131: 741-750.
Kalmar, I.D., D. Vanrompay, G.P. Janssens (2013) Broiler ascites syndrome: collateral damage from efficient feed to meat conversion. Vet. J. 197: 169-174.
Minne, B., E. Decuypere (1984) Effects of late prenatal temperatures on some thermoregulatory aspects in young chickens. Arch. Exp. Vet. Med. 38: 374-383.
Nassar, M., I. Halle, A. Plagemann, B. Tzschentke (2014) Detection of long-term influence of prenatal temperature stimulation on hypothalamic type-II iodothyronine deiodinase in juvenile female broiler chickens using a novel immunohistochemical amplification protocol. Comp. Biochem. Physiol. Part A 179: 120–124.
Nichelmann. M., B. Tzschentke (2002) Ontogeny of thermoregulation in precocial birds. Comp. Biochem. Physiol. A 131: 751-763.
Nichelmann. M., B. Tzschentke (2003) Efficiency of thermoregulatory control elements in precocial avian embryos (Review). Avian Poult. Biol. Rev. 14: 1-19.
Nichelmann, M., B. Lange, R. Pirow, J. Langbein, S. Herrmann (1994) Avian thermoregulation during the perinatal period. In: Zeisberger, E., E. Schönbaum and P. Lomax (Eds.) Thermal Balance in Health and Disease. Advances in Pharmacological Science, Birkhäuser Verlag AG, Basel, Boston, Berlin, ISBN 3-7643-5054-7, ISBN 0-8176-5054-7, 167-173.
OECD/Food and Agriculture Organization of the United Nations (2014), OECD-FAO Agricultural Outlook 2014, OECD Publishing. http:// dx.doi.org/10.1787/agr_outlook-2014-en.
Sallagundala, N., K. Yakimova, B. Tzschentke (2006) Characterization of neuronal hypothalamic plasticity in chicken: a comparative analysis. In: S. Yahav and B. Tzschentke (Eds.) New insights into fundamental physiology and peri-natal adapation of domestic fowl, eds. Nottingham University Press, ISBN 1-904761-44-5, 99-108.
Schmidt, C.J., M.E. Persia, E. Feierstein, B. Kingham, W.W. Saylor (2009) Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult. Sci. 88: 2610-2619.
Tzschentke, B. (2007) Attainment of thermoregulation as affected by environmental factors. Poult. Sci. 86:1025–1036.
Tzschentke, B., M. Nichelmann (1999) Development of avian thermoregulatory system during the early postnatal period: development of the thermoregulatory set-point. Ornis Fenn. 76: 189-198.
Tzschentke, B., D. Basta (2002) Early development of neuronal hypothalamic thermosensitivity in birds: influence of epigenetic temperature adaptation. Comp. Biochem. Physiol. A 131: 825-832.
Tzschentke, B., A. Plagemann (2006) Imprinting and critical periods in early development. World´s Poult. Sci. J. 62: 626-637.
Tzschentke, B., I. Halle (2009) Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Brit. Poult. Sci., 50: 634-640.
Tzschentke, B., M. Rumpf (2011) Embryonic development of endothermy. Respir. Physiol. Neurobiol. 178: 97-107.
Tzschentke, B., S. Tatge (2012) Embryonic temperature training for robust chicks. World Poultry 3 (28): 8-10.
Tzschentke, B., I. Halle, M. Nassar (2014) Temperature training in late-term quail embryos has transgenerational effects. In: Svihus, B. (Ed.), Proceedings, XIV European Poultry Conference, Stavanger, Norway, 510.
Tzschentke, B., S. Bogatyrev, K. Schellong, R.C. Rancourt, A. Plagemann (2015a) Temporary prenatal hyperglycemia leads to postnatal neuronal ‘glucose-resistance’ in the chicken hypothalamus. Brain Res. 1618: 231-240.
Tzschentke, B., I. Halle, M. Boerjan, L. Schrader, S. Tatge, S. Noack, S. Bogatyrev, W. Kloas (2015b) Short-term temperature training in the hatcher. International Hatchery Practice 30 (2): 27.
 Linkedin
Linkedin







