Abstract
Modern hybrid laying hens can produce more than 300 eggs per year and more than 500 eggs in an extended life cycle. This would not be possible without continuous ovulation, a trait already observed in the ancestors of our modern layers. In the ovary of newly hatched hens more than 12.000 oocytes are present. Of these, only a small proportion will later acquire yolk and develop into mature ovulatory oocytes. With the onset of sexual maturity follicles with different sizes develop by accumulation of lipids resulting in a hierarchy of follicles which ensures the development of only one pre-ovulatory follicle per day. The precise regulation of follicle selection is only partially understood but the pituitary hormones FSH and LH play a dominant role in this process. In contrast, formation of the yellow yolk is well described. With the exception of immunoglobulins all proteins and lipids are synthesized in the liver and transferred from blood stream into the developing follicle. Yolk contains approximately 36% lipids, 17% protein (largely immunoglobulins) and only a minor fraction of free carbohydrates and inorganic components (-1%), the rest is water. Maternal antibodies in the yolk are exclusively of the IgY type. They are made by B-lymphocytes in the spleen and other lymphoid organs, secreted into the circulation and actively transported in the ovary into the yolk. Yolk formation is completed 24 hours before ovulation.
In recent years proteomics have been used intensively to unravel proteins involved in the formation of the different egg membranes, albumen and the eggshell and identified novel proteins protecting the egg and the embryo from pathogens. In addition, new transcriptomic data help to better understand the regulation of egg formation still only partially understood.
After ovulation the follicle is placed in the infundibulum where the vitelline membranes are formed during the 1 hour passage. They separate the yolk from the album which is secreted by glandular and goblet cells of the magnum during the following 2-3 hours. 90% of the dray matter of the albumen is protein which is exclusively produced by the cells of the magnum. With the passage into the isthmus formation of the eggshell membranes starts which is followed by formation of the eggshell in the uterus. This process is controlled by the ovulation and several neuroendocrine factors which regulate the secretion of osteopontin and the expression of calcium transport proteins and calbindin. Within 11-15 hours 2.0-2.5 g of calcium are deposited in the eggshell. Two third of the daily calcium requirement is derived from intestinal resorption the remaining third is mobilized from unique bone structures called the medullary bone. This bone compartment is restored during the periods between eggshell formation. As in mammals, calcium homeostasis is regulated by 1,25-dihydroxyvitamin D3 and parathyroid hormone.
In recent years proteomics have been used intensively to unravel proteins involved in the formation of the different egg membranes, albumen and the eggshell and identified novel proteins protecting the egg and the embryo from pathogens. In addition, new transcriptomic data help to better understand the regulation of egg formation still only partially understood.
Keywords
chicken egg, production, egg structures, ovary, reproductive tract
Introduction
Modern hybrid laying hens can produce more than 300 eggs per year and more than 500 eggs in an extended life cycle. This would not be possible without continuous ovulation, a trait already inherited in the ancestors of our modern layers (1). In the ovary of newly hatched hens more than 12.000 oocytes are present. Of these, only a small proportion (250-500) will later acquire yolk and develop into mature ovulatory follicles to give rise to egg formation (2). Here I will briefly describe the stepwise development of the main structures of the chicken egg in the ovary and during its passage through the reproductive tract.
Follicle maturation
With the onset of sexual maturity follicles with different sizes develop by accumulation of lipids resulting in a hierarchy which ensures the development of only one preovulatory follicle per day. Sexually mature hens harbor numerous primordial follicles in the cortex of the ovary with up to 1 mm in size. This resting pool of follicles gives rise to a restricted number of primary follicles which further mature into so called pre-hierarchal follicles from which only one follicle (pre-ovulatory) is selected to terminally mature per day (3). The regulation of this selection process is only partially understood. It is assumed that inhibitory signals prevent the expression of the receptor for follicle stimulating hormone (FSH) which is secreted from the pituitary gland. Loss of this inhibitory signal in individual follicles leads to FSH-receptor expression and FSH mediated synthesis of steroid hormones and factors supporting survival of follicle associated cells. Subsequently, the FSH effect diminishes and luteinizing hormone (LH; another pituitary hormone) regulates further follicle maturation and accumulation of yolk. Initially, primordial follicles are recruited to the cortical surface and eventually become connected to the blood supply and nerve system via the follicle stalk. Maturation from primordial follicles via primary follicles into pre-hierarchal follicles is associated with accumulation of white yolk rich in lipoproteins. These follicles are 6-8 mm in diameter and can easily been seen on the ovary surface. The terminal differentiation of such follicles to the pre-ovulatory follicle takes 4-6 days and is characterized by the massive accumulation of yolk (2g per day) a process completed 24 hours prior to ovulation (4).Yolk composition
Yolk mostly consists of fat (36%), protein (17%) and water; 65-70% of the fat fraction are triacylglycerols, 25-31% phospholipids and 4-5% cholesterols. The protein fraction is composed of α-livetin (14%; = serum albumen), β-livetin (41%; = glycoproteins) and γ-livetin (45%; = IgY). Carbohydrates, vitamins and inorganic compounds represent less than 1% of the yolk. The characteristic color of the yolk is caused by carotenoids, in particular by xanthophylls (2). Both yolk fats and yolk proteins are synthesized in the liver a process induced by estrogen and testosterone secreted from maturing follicles in the ovary. The fat fraction is transported in the blood as very low density lipoproteins (VLDL). VLDLs bind to a specific receptors called LR8 which is highly expressed on the oocyte membrane and are subsequently endocytosed into the yolk. This receptor has been studied in some detail. In addition to VLDL it transports proteins and protein associated vitamins. Mutations of the LR8 receptor have been observed in hens causing hyperlipidemia and a loss of fat transfer into the oocyte leading to infertility (5).Immunoglobulin Y (IgY) is the only major yolk protein not being synthesis in the liver. It originates from plasma cells in lymphoid tissue which differentiate from B-lymphocytes during immune responses (6). IgY is actively transported from the blood into the oocyte a process mediated by a specific receptor in the oocyte membrane. The receptor is a member of the mannose receptor family and called FcRY (7). As a consequence of the receptor mediated transfer IgY composition in the yolk precisely reflects blood IgY composition. Up to 100 mg of IgY can be found in the yolk which provides maternal antibodies to the developing embryo and hatchling. In addition, this IgY is a rich source of antibodies for diagnostics and therapy.
Ovulation
15-60 minutes after oviposition the next mature follicle ovulates. Thus, the ovulation cycle takes nearly 24 hours in modern layer lines while it may take up to 27 hours in low producers. Ovulation is preceded by a surge of LH levels in the blood 4-6 hours prior to ovulation which is induced by increasing blood levels of progesterone secreted from the pre-ovulatory follicle. LH regulates the breakdown of connective tissue in the stigma area of the follicle, rupture of the follicle wall and release of the oocyte. The entire process of follicle maturation, hierarchal selection and ovulation has been studied in great detail and more comprehensive reviews including detailed descriptions of regulatory networks can be found in (2,4).A consequence of the high ovulatory rate in layers is the frequent formation of ovarian tumors. 30-35% of hens older than 2.5 years develop adenocarcinomas (8). Since layers are usually not raised to this age ovarian cancer is rarely seen under commercial conditions.
Formation of the egg in the reproductive tract
Rupture of the follicle wall leads to the release of the ovum which is captured by the infundibulum and thus funneled into the reproductive tract (Figure 1). Fertilization, formation of the vitelline membrane and of the first layer of albumen of the egg takes place in the infundibulum. The majority of albumen is produced during the passage through the magnum and subsequently egg shell membranes are formed in the isthmus. The final step of egg formation, the production of the egg shell, takes place in the uterus or shell gland before the egg is released by oviposition. This entire process takes 24-27 hours with most of the time required for shell formation. The structures of the egg are shown in figure 2. As discussed, 15-60 minutes after oviposition the next ovulation takes place.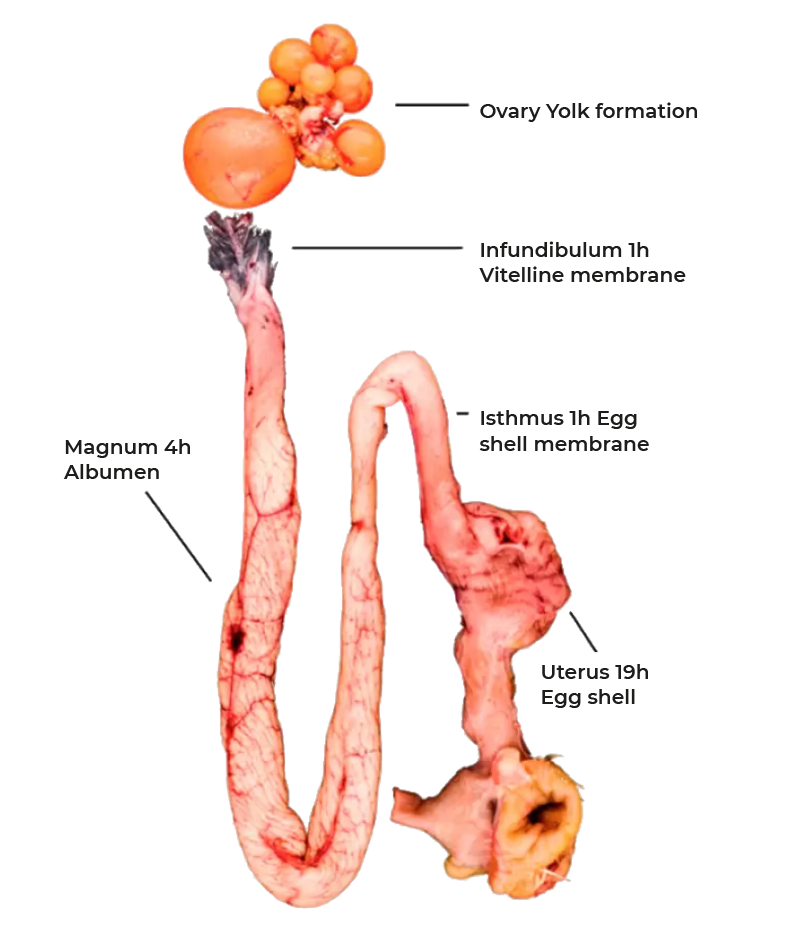
Figure 1: The reproductive tract of the chicken, stepwise egg formation.
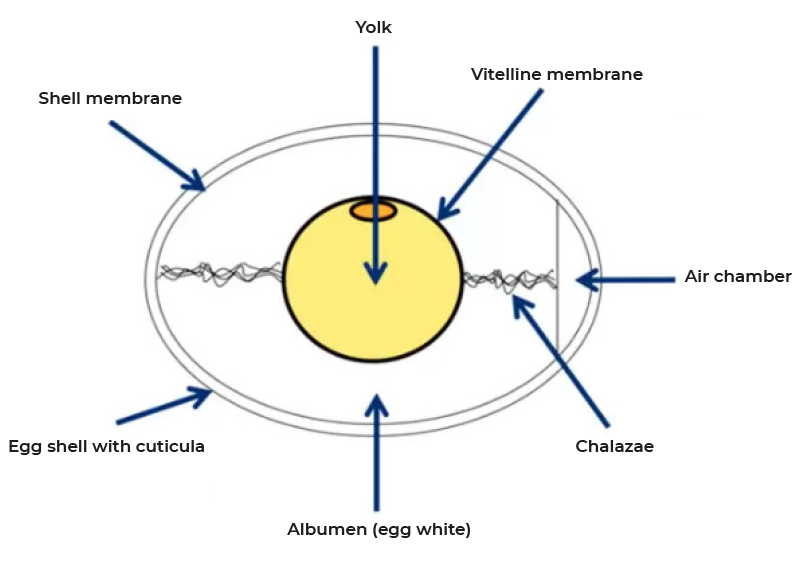
Figure 2: Structures of the chicken egg
Infundibulum
The ovum resides up to one hour in the infundibulum. During this time a membrane similar to the zona pellucida of mammals is formed which separates the ovum from the albumen. Recent studies have shown that at least 137 proteins are involved in the formation of the vitelline membrane most of them with unknown functions. However, it is known that it contains high concentrations of antimicrobial peptides (ß-defensin, lysozyme, proteases), thus forming an effective inner barrier against infection (9).Magnum
Subsequent to the infundibulum the ovum moves through the magnum where albumen formation proceeds during the next 3-4 hours. The main constituent of albumen is water with 88%. 90% of the dry matter are proteins, 6% are minerals and 3.5% is free glucose. In contrast to the yolk proteins, proteins of the albumen are not produced in the liver but by the mucosa of the magnum. The tubular glands secrete ovalbumin, ovotransferrin, ovomucoid and lysozyme, while goblet cells provide ovomucin and avidin. Synthesis of these proteins is regulated by estrogen and progesterone and continues even in the absence of a forming egg. In contrast, release of the preformed proteins is induced by mechanical stimulation as the forming egg moves down the magnum and is independent of endocrine or neuronal regulation. Albumen is well known for its antimicrobial properties caused by numerous peptides including lysozyme, avidin, cystatin and several defensins (10). At the same time chalazae form a process that starts early in the infundibulum. They are made up of protein fibers which connect the yolk and the shell membrane and thus ensure the central position of the yolk. Due to the rotation of the forming egg during the passage down the magnum the fibers wind to form the characteristic structure.The albumen is not homogenous but forms areas of different viscosity with a more liquid inner egg white, the thick egg white and chalazae and an outer more liquid layer. This final structure develops as the albumen becomes increasingly hydrated in the distal part of the magnum and the uterus a process mediated by solute secretion (sodium, calcium, magnesium and chloride) and passive water movement.
Isthmus
After transfer into the isthmus formation of the inner and outer shell membranes by a mesh of protein fibers is initiated. These membranes are closely spaced for the most part of the egg but segregate at the blunt end to form the air chamber. While they are impermeable for egg white they permit the exchange of water, gas (in particular oxygen) and minerals. On their outer surface projections form which are called mammillary cores made up of proteins, carbohydrates and mucopolysaccharides. It is these structures where the formation of the egg shell is initiated.Uterus (shell gland)
With the arrival of the egg in the uterus hydration of the egg white continues. Water is pumped across the shell membranes which lead to a volume increase and the development of the characteristic ovoid form. Consequently, the forming egg gets in close contact with the uterine wall permitting efficient formation of the egg shell. This process takes up to 19 hours and is characterized by the secretion of an organic matrix of glycoproteins and mucopolysaccharides which becomes calcified to from the egg shell (11). Calcification requires approximately 2 to 2.5 g of calcium per egg which forms calcite crystals, a highly stable form of calcium carbonate (CaCO3). The crystalline structures grow up from the mammillary cores to form into highly organized palisades giving the unique strength to the egg shell. Layers producing 300 eggs per year require nearly 1.8 kg of calcium matching their own body weight. 60-80% of the calcium is absorbed from the intestine during shell formation if hens are provided with the recommended 3.6- 4% of calcium in the feed. The remaining 20-40% of calcium required is mobilized from the bone. Enteral resorption of calcium is regulated by 1,25-dihydroxyvitamin D3 while mobilization of calcium from the bone is under control of 1,25-dihydroxyvitamin D3 and parathyroid hormone (PTH). Calcium mobilized from the bone will be replenished when the uterus is empty and calcium absorbed in the gut is fully available for bone matrix formation. Importantly, hens develop a unique form of bone matrix called the medullary bone. Two weeks prior to lay a meshwork of interconnecting spicules develops under the control by estrogen and testosterone in the long bones. This structure accounts for up to 12% of the total bone calcium but provides little support for bone stability. However, mobilization of medullary bone is 10-times faster than that of cortical bone and thus can quickly make up for a lack of calcium resorption from the gut (4).To form the egg shell calcium and bicarbonate (HCO3-) must be secreted into the uterine lumen to form the calcite crystals (CaCO3). Calcium is taken up by epithelial cells in the uterus from the blood by a Ca2+-channel and transferred by a Ca2+ binding protein (calbindin 28K) to the apical membrane where it is actively pumped into the uterus lumen against a concentration gradient. Several Ca2+ transporters have been described and recent proteomic studies have added new candidates to the list (12).
To maintain a low Ca2+ concentration in the epithelial cytoplasm which is critical for cellular homeostasis Ca2+ is not only bound to calbindin but also transported into cellular storage compartments (endoplasmatic reticulum) from where it can quickly be released during active shell formation. HCO3- is generated from water and carbon dioxide (CO2) in the cell and transferred into the uterus lumen by a transporter secreting HCO3- and taking up Cl- in exchange. In the lumen Ca2+ and HCO3- react to calcite crystals to build the mineralized shell matrix.
The final step prior to oviposition is the deposition of a waxy membrane on the egg shell called the cuticula. It is composed of polysaccharides, lipids and more than 50 proteins many of them with antimicrobial activities. Accordingly, its primary function is to prevent penetration of microbes into the egg and to limit the loss of water from the egg (13).
Oviposition
This process is initiated by the relaxation of the uterine sphincter and the contraction of uterine smooth muscles. It is regulated by neurohypophysial hormones, primarily oxytocin and arginine-vasotocin as well as prostaglandins (e.g. PGF2α) synthesized locally within the reproductive tract. Within the next 60 minutes after oviposition the next ovulation will take place.Conclusions
Egg morphology, composition and formation have been studied intensively for more than a century and a large body of literature is available. However, many of the regulatory circuits are only partially understood. New technologies such as next generation sequencing and proteomics (12, 14, 15) may help to gain new insides into the multiple steps of egg formation and the precise composition of yolk, albumen, egg membranes and the egg shell. Reverse genetics are also progressing in the field of avian biology and should finally enable functional in vivo studies of selected genes (13).

References
1. Flock DK & Havenstein GB (2015) Demand Driven Productivity in the Egg Business: Combining Advances in Genetics, Health Control and Nutrition to Meet Changing Consumer Preferences. Lohmann Information 49(1), 30-37.2. Nys Y & Guyot N (2011) Egg formation and chemistry. Improving the Safety and Quality of Eggs and Egg Products, eds Nys Y, Bain M, & Van Immerseel F ( Woodhead Publishing), pp 83-132.
3. Johnson AL & Woods DC (2007) Ovarian dynamics and follicle development. Reproductive Biology and Phylogeny of aves, ed Jamieson BGM (Science Publishers Inc.), pp 243-277.
4. Johnson AL (2015) Reproduction in the female. Sturkie’s Avian Physiology, ed Scanes CG (Elsevier, Amsterdam), Sixth Ed, pp 635 – 665.
5. Nimpf J & Schneider WJ (1991) Receptor- mediated lipoprotein transport in laying hens. The Journal of nutrition 121(9):1471-1474.
6. Härtle S, Magor KE, Göbel TW, Davison F, & Kaspers B (2014) Structure and evolution of avian immunoglobulins. Avian Immunology, eds Schat K, Kaspers B, & Kaiser P (Elsevier, Amsterdam), pp 103-120.
7. Tesar DB, Cheung EJ, & Bjorkman PJ (2008) The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Molecular biology of the cell 19(4):1587-1593.
8. Johnson PA, Stephens CS, & Giles JR (2015) The domestic chicken: Causes and consequences of an egg a day. Poultry science 94(4):816-820.
9. Mann K (2008) Proteomic analysis of the chicken egg vitelline membrane. Proteomics 8(11):2322-2332.
10. Etches RJ (1996) Reproduction in Poultry (Wallingford; CAB International).
11. Nys Y, Hincke MT, Arias JL, Garcia-Ruiz JM, & Solomon SE (1999) Avian eggshell mineralization. Avian and poultry Biology Reviews 10:143-166.
12. Jonchere V, et al. (2010) Gene expression profiling to identify eggshell proteins involved in physical defense of the chicken egg. BMC genomics 11:57.
13. Schusser B, et al. (2013) Immunoglobulin knockout chickens via efficient homologous recombination in primordial germ cells. Proceedings of the National Academy of Sciences of the United States of America 110(50):20170-20175.
14. Mann K & Mann M (2008) The chicken egg yolk plasma and granule proteomes. Proteomics 8(1):178-191.
15. Mann K & Mann M (2011) In-depth analysis of the chicken egg white proteome using an LTQ Orbitrap Velos. Proteome science 9(1):7.