Summary
A commonly recognized problem of modern dairy farms is that the extremely high milk production
often leads to a shorter productive life so that it becomes difficult to maintain the herd size from own
replacement heifers. The herd management of a dairy farmer in Germany is described as a case
study to show that competitive family income can be achieved by focusing on a combination of high
milk yield, longevity and health of the cows instead of on maximum milk production at the expense
of other equally important traits. The practical experience of the owner family, focus on wellbeing of
the herd and health care for individual cows, a nutrition program designed for high intake of roughage
and continuous checking in the cow barn while the cows use the milking robot are seen as essential
factors in this successful dairy operation.

Introduction
Veterinary activity is focused simultaneously on the animal itself and the suitable environment. Animal care includes detailed diagnostics, prophylactic immunization and, in some cases at least, effective treatment. In the environment, practically all plants, animals and humans are colonized by bacteria, fungi, viruses, prions but also by single cell coccidia and multicellular parasites. As a logical consequence, a permanent fight exists to control such agents and their debilitating effects on animal health, welfare and productivity. Effective measures are essential to combat these pathogenic microorganisms with the target to reduce or even to eliminate their deleterious effects. Such measures are applied on non-living objects and generally summarized under the term “disinfection”.Highly desired effects of disinfection are (i) all pathogenic microorganisms, all oocysts of coccidia, all eggs of internal and external parasites are totally destroyed in all areas, (ii) re-introduction of pathogens is permanently prevented, (iii) losses due to transmissible diseases are minimized, (iv) profitability of animal farming is enhanced and (v) a positive cost-benefit balance is assured.
Proper disinfection is usually associated with undesired effects. These effects may be (i) irritation of skin and eyes of workers, (ii) slippery surfaces may cause accidents, (iii) persistence of disinfectants in rooms and on surfaces, (iv) some disinfectants discolour painted walls, (v) spill of disinfectant into the environment, open waters, creeks etc., (vi) toxicity for fish, crustaceans, arthropods, plants etc., (vii) damage to electrical wires, to engines, equipment and (viii) corrosion on metal surfaces.
Various definitions have been given to characterize “disinfection” and to discriminate it from related or similar measures. The following delineates some selected definitions.
Disinfection is a method applied to prevent transmission of disease causing agents (Schließer, 1981) procedure used for inactivation of certain microorganisms (Böhm & Straub, 2002) abolition (abrogation) of disease causing agents (Duden, 2006) method that destroys infection-producing agents (Blood et al., 2007) It is clear from these four selected definitions that the prevailing target is the elimination or at least reduction of such agents that (may) cause disease. It is never attempted to obtain an environment that is devoid of virtually all microorganisms. Also, the aim is prevention of transmission and infection by destroying the vital properties of pathogenic organisms.
The term “disinfection” requires demarcation from other more or less related technical means and procedures.
Some examples are:
cleansing means mechanical removal of dirt, manure, dust, vermin etc.
sterilization is the application of dry heat for several hours on solid materials
filtration means separation of organic matter in a liquid phase by membrane or asbestos filters of variable pore size
pasteurization includes repeated cycles of heating and cooling of liquid matter for variable times and temperatures
antisepsis is the application of germicidal substances on and in animals
On the history of disinfection
Some historical remarks should be added at this point on means to correct environmental pollution of air and surfaces and on preservation of human food. Elevated but appropriate room temperature for hatching chicks was achieved in old Egypt in so-called Pharao hatcheries by burning of plant material.The developing smoke contains phenolic compounds that condense at surfaces in the hatchery compartments and exert their disinfecting effects. Another example: The common plant thyme (Thymus vulgaris) is frequently used in herbal medicine. It contains high concentrations of thymol which is chemically o-isopropyl-m-cresol, a substance with a high disinfecting property. Thirdly, even in contemporary times smoke and smell are generated by smouldering of the resin derived from the tree Boswellia serrata which contains (among many others) a high concentration of phenolic substances and tare which have disinfecting properties and also emit a pleasant smell. Frankincense is commonly used in religious ceremonies e.g. in catholic churches – without realizing the real disinfecting effects of this practice.
I mention these practices to illustrate different germ reduction strategies which have been used for centuries without realizing the basic modes of chemical reaction and without realizing and using the contemporary term “dis-infection” which means the opposite of “infection”.
In the past and in some areas even today, various means are practised to maintain and to preserve edible food for human consumption. Such measures include reduction of the water content of food by drying and salting or by adding sugar or keeping food on ice.
Another example of traditional “disinfection” is the rather common use of sunshine to dry and bleach cloths etc. on green meadows. Housewives interpret this as an effective measure to turn the greyish appearance of cotton to splendid white and to obtain dry and nice smelling cloths. Actually, the most important effect of “drying” in sunshine is directly associated to inactivation of the infectivity of microorganisms. It is well known that UV-light destroys the genome of pathogenic microorganisms within a short time, and drying and bleaching are just welcome additional side effects.
Historically, the most important prerequisite of disinfection was the development of techniques for isolation and identification of microorganisms, especially bacteria and viruses. Pioneers in this field were Louis Pasteur (1822-1895) in France (Pasteurella spp.), Robert Koch (1843-1910) in Germany (Mycobacterium tuberculosis), Theodor Escherich (1857-1911) in Austria (Escherichia spp.), Georg Theodor August Gaffky (1850-1918) in Germany (Salmonella spp.) and Friedrich Loeffler (1852-1915) in Germany (Foot and mouth disease virus). These and many other outstanding scientists paved the way to microbiology and fundamentals of the theory of infectious diseases.
Equally important for the promotion of microbiology and disinfection were chemists who isolated and identified chemicals and developed techniques for their synthesis which might be useful as disinfectants.
Outstanding in this respect is August Wilhelm Hofmann (1818-1892) who discovered in 1867 (among many other inventions) the structure of formaldehyde and developed the synthesis of this chemical.
Initial studies on inactivation of microorganisms by available chemicals were undertaken some hundred years ago. Robert Koch was the first to test phenol for the inactivation of spores of Bacillus anthracis and published his results in 1884. He contaminated threads of silk by sporulated bacteria that cause anthrax, dipped these threads into a phenol solution and tried to re-isolate these bacteria after several time intervals. More recently, the German Society for Hygiene and Microbiology produced the first guideline in 1958 which contains already suspension and carrier tests and selected bacteria and fungi as test organisms.
Currently, the fourth edition of the guidelines provided by the German Veterinary Society (DVG) forms the basis for any testing of disinfectants in Germany. Besides suspension and carrier tests, a reference disinfectant and a reference test virus are included. All disinfectants must be examined by at least two independent persons who were approved by the board of the DVG. On an international level, various organisations are involved in the development of testing procedures for the evaluation of disinfectants.
In the following, past and current procedures for testing and evaluation of chemicals for disinfection in the veterinary field are reviewed. Various governmental and non-governmental organisations developed guidelines for this purpose. Internationally operating organisations include OECD, FAO, EFSA, FDA, national organisations include the British Standards Institute (BSI); in Germany the Robert-Koch-Institute (RKI), the Veterinary Society (DVG), the Society for Applied Hygiene (VAH) and the German Agricultural Society (DLG). National organisations for testing and evaluation of chemical disinfectants exist also in The Netherlands, France and Italy, to name only a few countries.
All these organisations developed procedures that are similar in testing principles, but the details of methodology, the agents used and the evaluation and subsequent recommendations differ quite markedly. Since 1989 the European Committee for Standardization (CEN) is working on harmonization of test procedures and their efficient evaluation of results.
The results obtained on the basis of a firmly established testing protocol are of paramount importance for the producer, for the testing establishment and finally also for the user of a disinfectant.
Producers wish to obtain (i) reliable and reproducible test results for efficacy and safety reasons, (ii) a low working concentration of a disinfectant because low working concentrations have a strong bearing on the market price and (iii) results that are acceptable in almost all countries in order to expand their international market without re-testing of their products in various countries. The users prefer a broad spectrum of efficacy at low cost. The testing laboratories need a testing procedure which (i) yields results that must be reproduced in their own and in other laboratories, (ii) is efficient and not too elaborative and (iii) can be obtained within acceptable times.
The sub-committee for disinfection of the German Veterinary Society (DVG)
Around 1970 Theodor Schließer in Giessen promoted the development of guidelines for testing of chemical compounds as disinfectants for virucidal, bactericidal, levoricidal and antiparasitic efficacy.The initial testing protocols were further refined during the following decades. Currently, the fourth edition (2004) of the guidelines for testing and evaluation of chemical compounds for application in the fields of veterinary medicine and food production are in use. Both guidelines require suspension and carrier tests and contain lists of reference bacteria, viruses, fungi and parasites. The results of testing that were generated by two laboratories are evaluated by the sub-committee and subsequently published at regular intervals in the Deutsches Tierärzteblatt and are accessible on the internet.
Two separate lists are currently produced. One list contains data on disinfectants intended for use in the field of animal production; the second list aims at use in food hygiene. Both lists contain all essential background information (concentration, time, temperature) and the proven efficacy against viruses, bacteria, fungi and parasites.
The European Committee for Standardization (CEN)
This Committee started its work in 1989 during the first meeting in the building of the British Standards Institution (BSI) in London, UK. The committee is composed of a maximum of three delegates per European country. In Germany, the delegates are selected and approved by the German Institute for Standardization (DIN) in Berlin.The CEN has the following structure:
Technical Committee TC 216 – evaluates content and form of all drafts for standards and makes final decisions. The TC 216 inaugurates and installs three working groups:
Working group 1 is responsible for drafts on standardization in human medicine
Working group 2 is responsible for drafts on standardization in veterinary medicine
Working group 3 is responsible for drafts on norms in areas of food, drinks, cosmetics, hospitals, kitchens etc.
The Technical Committee 216 may create Special Task Groups for defined topics and workloads such as “surface test task group”, “sporocidal test task group” or “virucidal task group”. Such groups have to perform specified experiments and to report on the results to the Technical Committee 216 within a fixed deadline.
General duties of the CEN are to further improve guidelines that contain detailed prescriptions for test organisms (including proven purity, identity and infectivity titres), necessary diluents for test organisms, materials, size and surface structure of germ carriers (e.g. poplar or linden wood, steel, glass, plastics etc.).
Even now a large number of questions on the testing procedure are still open to debate. In order to answer at least some of the very pressing questions, members of the Committee are asked to perform specified experiments “at home” and report the obtained results to all members of the Committee prior to each session, to comment on the results in writing and answer questions during the sessions.
It appears that all members consider this procedure as essential to obtain further progress.
The content and structure of the testing protocol is also important. Required are data on the method used, the microorganisms, the organism and disinfectant used as references.
So far, the CEN produced a large number of preliminary reports. The circulated drafts are termed “preliminary European Norm, preEN” and an approved final text represents a European Norm (EN).
Quite a number of such ENs exist already and are in full use in Europe. Comprehensive texts of all approved EN are available on the internet.
At any time in the future the work on standardization of testing of disinfectants and evaluation of procedures for disinfection will be completed and all results are then published. At this future stage further work is planned for harmonization of aims and means in cooperation with non-European countries (e.g. USA, Japan, India) and internationally operating organisations such as OECD, FAO, WHO.
Areas that may need disinfection
Practical experience and solid scientific evidence prove the existence of highly different microbial populations at different locations. In the veterinary field, such locations require different disinfectants and carefully selected modes of application.Such locations may be:
• Stables, including roads and gateways
• Liquid and solid manure
• Hatching eggs, hatcheries, chick trucks
• Slaughterhouse, transport vehicles
• Milk production: teats, tubes, vessels
• Breweries: in process applications
• Hands, towels, offices, door locks, …
• Oil production and conservation
• Machineries lubricating oils, cooling devices, …
These and most likely other “locations” require correct disinfection in specified situations. The selection of the most appropriate chemical disinfectant for any contaminated location leads to the question of prevalence of specific pathogenic microorganisms and available chemical compounds.
Microorganisms as test models
Two conditions must be considered for the use of a microorganism for testing purposes. These are the multiplication of an organism to high concentrations (titres) under laboratory conditions and the relative resistance to chemical inactivation which is known from the results of previous experiments. After long debates during committee meetings of the DVG, DIN and CEN the following mandatory test organisms were approved and published in several EN for quantitative suspension and carrier tests:Bacteria: Pseudomonas aeruginosa, Proteus vulgaris, Enterococcus hirae, Staphylococcus aureus
Mycobacteria: Mycobacterium avium
Levuricidy: Candida albicans, spores of Aspergillus niger
Viruses: Newcastle disease virus, vaccinia virus, reovirus, bovine enterovirus type 1, others if desired
Parasites: Ascaris suum eggs, Eimeria tenella oocysts
In addition to these approved test organisms additional agents of interest may be tested on an optional basis. More recently, these are avian influenza A viruses (subtypes H5N1, H1N1, H6Nx H9Nx) West Nile virus, calicivirus, strain Norwalk. For these and any other optional viruses the CEN-approved testing procedure must be followed. The general pathway consists of the following phases and steps:
• Phase 1: laboratory testing of chemicals for antimicrobial efficacy
• Phase 2, step 1: quantitative suspension test
• Phase 2, step 2: quantitative carrier test
• Phase 2, step 3: quantitative surface test under field conditions
Basic tests on chemical disinfectants
All active substances intended for use in disinfectants must be listed in Annex I or IA to Directive 98/8/EC. The commercial product is classified as product type 3 (Veterinary hygiene biocidal products).For conformity, the following information and material is required:
• Chemical composition of the disinfectant, identity and quantity of the active substance, minimum purity of the active substance, absence of impurities.
• Physical and chemical properties (e.g. pH at various concentrations in water of standardized hardness; solubility in water of standardized hardness)
• Freedom of contamination by infectious agents and other cell type of primary or permanent cell cultures. Permanent cell lines can be obtained from the American Cell Culture Collection, Manassas, Virginia, USA, the Cell Culture Bank, FLI, Insel Riems or from well-known virological laboratories. Primary cell cultures are produced from SPF chicken eggs, VALO, Cuxhaven.
• Pure and well characterized microorganisms (viruses, fungi and bacteria) are maintained and distributed by National Reference Laboratories, the American Type Culture Collection, and in Germany by the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig. Additional sources are published by the Deutsche Veterinärmedizinische Gesellschaft e. V., Giessen in the 4th edition of the “Richtlinien für die Prüfung chemischer Desinfektionsmittel”.
• There is no formal source for ascaride eggs. These eggs are collected from the intestine of pigs at slaughter. Oocysts of Eimeria tenella, strain Houghton, are maintained and distributed on formal request by the Institute for Animal Health, Compton Laboratories, UK.
• Toxicological profile for man and animals including metabolism (e.g. determined cytotoxicity in approved cell cultures)
• Effectiveness against target organisms (e.g. minimal inhibitory concentration of test microorganisms)
• Ecotoxicological profile including environmental fate and behaviour
• Measures necessary to protect man, animals and the environment
If these conditions are fulfilled, a more specific question arises which refers to the intended uses of disinfection. Under field conditions, the following groups of pathogens are of major interest:
• Bacteria including Chlamydia spp. and Mycoplasma spp.
• Viruses (and possibly prions)
• Protozoa: coccidial oocysts, flagellates (e.g. Histomonas spp., Trichomonas spp.)
• Eggs of round and tape worms
The inherent biological properties of these organisms vary extremely from highly sensitive to highly resistant. Therefore, the disinfectant must be tailored according to the predominant and mostly prevalent target microorganisms. In other words, a meaningful selection of an available disinfectant is necessary
Chemicals useful as disinfectants
Surprisingly, all currently used chemical disinfectants are “old” compounds and their chemical actions are known for many decades. These chemicals include:• Aldehydes – formaldehyde, glutaraldehyde, glyoxal
• Aliphatic alcohols – ethanol, propanol, isopropanol
• Aromatic alcohols – phenols including alkyl-, aryl- and halogen derivates
• Organic acids – formic, acetic, propionic, citric acid
• Hydrogenperoxide – H2O2
• Guanide – biguanide
• Iodophors – alcoholic solution of potassium iodide
• polyvinylpyrrolidone solutions of iodine
• Chlor – organic and inorganic compounds
• Alkalines – NaOH, CaO, Ca(OH)2, bleach
• Peroxyacetic acid (plus alcapur)
• Quaternary ammonium compounds
Some of these chemicals are now considered as obsolete; some others are applied under limited conditions. Beside a reliable mode of fast action, presently more impetus is given to the fate of a disinfectant after its application. Desirable are (i) rapid degradation without residues in the area of application, (ii) low level of toxicity to farmed animals and men, (iii) easy to handle during application, (iv) effective across a wide range of low and high environmental temperatures and (v) low price.
In recent years organic acids and especially peroxyacetic acid (plus alcapur) apparently gained more interest and widespread use under farm conditions. Various phenol derivates maintain a strong position as antiparasites. Almost all commercially available products contain not only an active ingredient, but also additives to enhance dispersion on surfaces (surface-active compounds).
Formalin as a traditional disinfectant
Formaldehyde is the gaseous form and its solution in water is named formalin. Formalin is on the market either at a concentration of 35 – 37% or as crude formalin at various concentrations. Since the discovery of formaldehyde by A. W. Hofmann in 1867, this compound was recognized as a disinfectant of superior value. The aqueous solution of formaldehyde was initially used to prevent fouling of animal skins prior to tanning. It was soon realized that repeated formalin exposure of the skin of tanners resulted in hypersensitivity, inflammation and pruritus. Since these early observations, formalin is considered as a potent allergenic compound. It does cause epithelial hypertrophy in the respiratory tract of rats that were experimentally exposed to high concentrations for prolonged times.More recently rumours spread that formalin may cause also cancer in experimental animals. Definite proof for this assumption is still lacking. However, the Institute for Risk Assessment in Berlin argues that a “certain risk for cancer development cannot be excluded.”
Despite this debate with pros and cons, formalin experienced a world-wide use as topical disinfectant especially for the disinfection of shells of hatching eggs. Rather recently, formalin was replaced by other compounds in hatcheries for safety reasons. It seems to be appropriate to list some of the major facts on formalin:
• 1867: first synthesis by August Wilhelm Hofmann in Giessen, Germany
• Technical synthesis today in Germany: approx. 500,000 metric tons per year by catalysis: CO2 + H2O = HCHO
• Automobiles in Germany emit ca. 35,000 tons CO2 per year (2004 statistics)
• Naturally present in plants, cigarette smoke and exhaust of gasoline and diesel engines
• Overwhelming amount is used for the production of plastics, waxes, glues, insulation foams for buildings, panel wood etc.
• Small fraction of the total production is used in medicine, anatomy, histology, pathology, production of inactivated vaccines, cosmetics, deodorants, creams …
• Polymerisation of formaldehyde to paraformaldehyde, so-called „dry alcohol“ is used by campers
• Maximal concentration at working places is fixed by the German Institute for Risk Assessment, Berlin, at 0.6 mg/m³, equal to 1 ml/m³, equal to 0.5 ppm. Experienced people cannot smell this level
To complete the list of known facts on advantages and disadvantages of formalin, the following should be kept in mind:
Advantages of using formalin as a disinfectant are the following facts:
• Effective at rather broad range of temperatures
• Effective against bacteria, fungi, yeasts, some viruses
• Effective in liquid and gaseous forms
• Effective at pH 4.0 to 9.0
• Penetrates porous surfaces, e.g. egg shells
• Oxydation to formic acid: 2 HCHO + O2 = 2 HCOOH
• Production is cheap, favourable cost-effect ratio
Major disadvantages of formalin are:
• Not listed in Annex I and IA of the Biocide Directive 98/8/EC. Legal use in the fields of medicine and animal production is not permitted anymore or requires special permit
• Causes irritation of conjunctiva and respiratory tract of humans and animals
• Causes allergic contact dermatitis after prolonged exposure
• Evaporates for long times from treated wood, panel wood, furniture etc.
• Hardens plastics – electrical cables etc.
• Polymerisation is prevented by methanol which is highly toxic
• Is inactivated by proteins
• Assumed to cause nasopharynx carcinoma after long-time exposure of rats
These and possibly more advantages and drawbacks may exist in various fields of technical and biological applications. In any case, it is worthwhile to look for promising alternatives to formalin as chemical disinfectants.
Detection of formaldehyde by its smell
Some people maintain that they are able to recognize the rather characteristic smell (odour) of formaldehyde even at extremely low levels, e.g. evaporating from newly acquired furniture made of wood panel. These people consider formaldehyde as a dangerous product to their health and wellbeing. In an attempt to confirm the ability to detect this gas, I asked my veterinary students to determine the lowest concentration which they are able to detect by smelling briefly opened petri dishes. For this purpose dilutions of formalin were produced in distilled water in the range of 10-2 to 10-5 and two millilitres of each of the dilutions were placed on filter paper in closed petri dishes. A separate petri dish was offered to the students that contained two millilitres of distilled water. Beginning with the highest dilution of formalin, the students were asked to write a protocol on their ability to detect the smell of formaldehyde in each of the petri dishes. This experiment – performed on a voluntary basis – was done with veterinary students of the fifth and seventh semester. The results are presented in the following Tables 1 and 2.Table 1: Recognition of formaldehyde (FA) by 44 veterinary students, 5th semester.
MAC 0.6mg/m³ ( = 1 ml/m³ = 0.5 ppm). N. t. = not tested
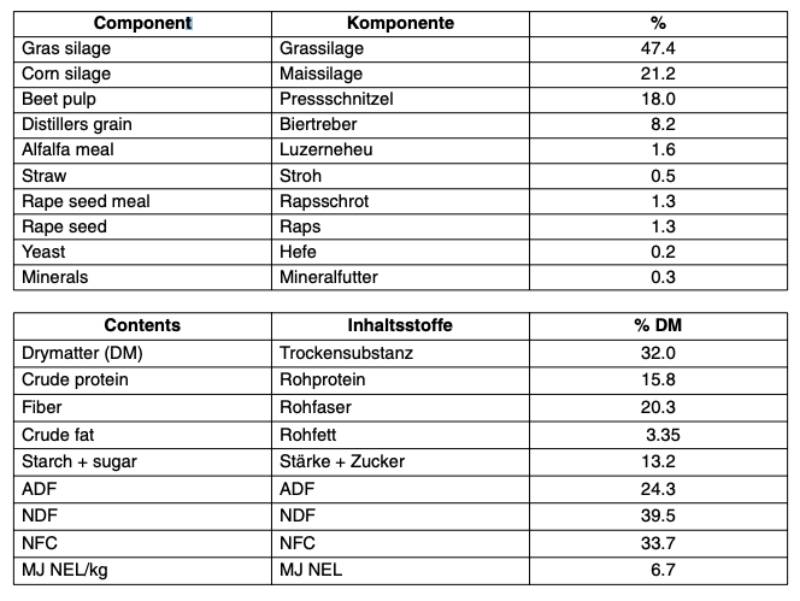
In medicine, anatomy, histology and pathology, phosphate buffered formalin is generally used in concentration between 9% and 10%. All students detected in correct manner formaldehyde at concentrations of 3.7 and 0.37%. However, lower concentrations were not recognized by all students. The maximum working concentration (MAC) of formaldehyde is in Germany fixed at 0.5 ppm. Obviously, approximately half of the students detected this low level by smelling. Some of them believed that they smelled the odour of formaldehyde even in distilled water. Thus, the errors of correct detection increased at low levels.
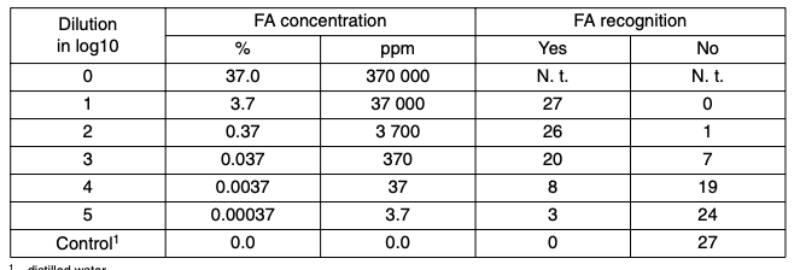
The same arrangement of the test was repeated with students of the seventh semester. The results are quite similar to the first test. Again, high concentrations were recognized in a correct manner. At low concentrations the number of students increased that came up with obviously wrong results. A few students (3 of 27) recognized formaldehyde at a concentration of 3.7 ppm. In contrast to the first test, all students interpreted the control sample that consisted of distilled water in a correct manner. In conclusion, formaldehyde at levels well above to the MAC value can be detected only by a few students but the rates of failure are high and therefore unreliable.
Table 2: Recognition of formaldehyde (FA) by 27 veterinary students, 7th semester.
MAC = 0.6mg/m³ ( = 1 ml/m³ = 0.5 ppm). N. t. = not tested
Alternative approaches for the inactivation or elimination of pathogens
Ironically, nothing – including pathogens – live for ever. This common saying applies also for pathogens.An inactive approach to get rid of pathogens according to the advice “just wait, time helps” may be effective under certain circumstances. However, under modern life style conditions active actions are generally preferred. Almost no commercial, but mainly hobby-type activities were and are practised in some selected alternative areas for the inactivation or elimination of pathogens. These may include the following:
Biologically:
composting of carcasses production of biogas addition of harmless bacteria to decaying plants and carcasses addition of cultivated earth worms (Eisenia foetida or Lumbricus terrestris) or fungi
Physically:
enhanced heat generation during composting Heat plus elevated pressure (autoclave) Sunshine or UV light Gamma radiation Chemically:
Oxidation by exposure to open air Denaturation by addition of lime stone or calcium hydroxide These measures have their own merits and under certain circumstances and meaningful use in both, under extensive poultry production in developed and in developing countries with small scale productivity. At least some of these germ reduction strategies will still be applied in the future. Major applications of disinfection are established following approved testing of efficacy and likely side-effects. Such testing is performed in suspension and germ carrier tests. This is demonstrated by the methodology provided by the German Veterinary Society.
Quantitative virucidal suspension test
The quantitative virucidal suspension test includes• Four test viruses: cytopathogenic Newcastle disease virus (NDV), vaccinia virus, strain Elstrey, human reovirus type 1, enteric cytopathogenic bovine orphan virus (ECBOV)
• Several temperatures, at least recommended 4 and 10°C
• With protein (40% foetal calve serum, FCS) and without protein loadv
• Several concentrations of disinfectant under test
• Quantitative determination of residual virus by titration
• Minimum requirement to pass: at least 4 log10 reduction as compared to control
Each assay has to be performed in duplicates and the final report must contain details of all methods and a recommendation for practical use. The quantitative virucidal suspension test is considered as an initial test to provide orientation of the efficacy of the tested disinfectant. A quantitative carrier test must follow.
Quantitative virucidal carrier test
The major aim of the quantitative virucidal carrier test is to confirm or to disprove the results of the suspension test. It consists of the following components:• Four test viruses: cytopathogenic Newcastle disease virus (NDV), Vaccinia virus, strain Elstrey, human reovirus type 1, enteric cytopathogenic bovine orphan virus (ECBOV)
• Carrier: mandatory is poplar wood, optional are linden wood, steel, concrete, others
• Mandatory are temperatures of 4 and 10°C, optional are several other and room temperatures
• Required is a protein load of 40% fetal calve serum (FCS)
• Several concentrations of disinfectant under test
• Quantitative determination of residual virus by titration
• Minimum requirement to pass: at least 4 log10 reduction as compared to control
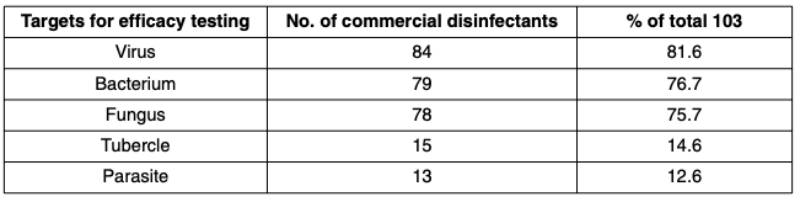
Number of listed disinfectants per indication in 13th list of DVG, 2011
The sub-committee of the DVG on disinfection in the veterinary field, division animal farming, approved in 2011 a total of 103 commercial products (Table 3). According to the intension of the producers, these disinfectants were not examined for efficacy against all test organisms. Most of the commercial disinfectants (84 products, 81.6%) were examined for virucidal efficacy. Second and third rank bactericidal and fungicidal efficacy. Rather few products were examined for their tuberculocidal and antiparacitic efficacy.The active compounds of disinfectants intended for use in the veterinary field (animal production) are contained in Table 4. The peroxyacetic acid and combinations of this acid with other organic acids is the most frequently listed compound for the field of animal farming. Second rank aldehydes (various combinations that were not specified in the 13th list) either as mono preparation or in combination with quaternary ammonium compounds (QUAC) and alcohols. Various aldehydes – not specified in the 13th DVG list – represent also a major group of disinfecting compounds (Table 4). Aromatic circular hydrocarbons such as derivates of phenol and cresol find their prevailing applications for disinfection of parasites (eggs of round- and tapeworms and oocysts of Eimeridae). The organic acids, formic and acetic acid in particular, are now more frequently listed as compared to previous lists.
Table 3: Number of commercially available chemical disinfectants for use in the veterinary
field according to targets of their intended use (13th DVG-list, published in 2011)
References
ADR (2012): Arbeitsgemeinschaft Deutscher Rinderzüchter. Rinderproduktion in Deutschland 2011. ISSN 1439-8745.ALVÅSEN, K., MÖRK, M., SANDGREN, H., THOMSEN, P. T., EMANUELSON, U. (2012): Herd-level risk factors associated with cow mortality in Swedish dairy herds. J. Dairy Sci. 95, 4352-4362.
BAIRD, G. D. (1982): Primary ketosis in high-producing dairy cow: Clinical und subclinical disorders, treatment, prevention, outlook. J. Dairy Sci. 65, 1-10.
BERRY, D., HORAN, B., DOBOVAN, M., MUCKLEY, F., KENNEDY, E., McEVOY, M., DILLON, P. (2007): Genetics of grass dry matter intake, energy balance, and digestibility in grazing Irish dairy cows. J. Dairy Sci. 90, 4835-4845.
BERGK, N., SWALVE, H. (2011): Der Fett-Eiweiß-Quotient in der Frühlaktation als Indikator für den Verbleib von Erstkalbinnen in der Milchkuhherde. Züchtungskunde 83, 89–103.
BICALHO, R., MACHADO, V., CAIXATA, L. (2009): Lameness in dairy cattle: A debilitating disease or a disease of debilitated cattle? A cross-sectional study of lameness prevalence und thickness of digital cushion. . J. Dairy Sci. 92, 3175-3185.
BUTLER, W. R. (2003) Energy balance relationships with follicular development, ovulation und fertility in postpartum dairy cows. Livestock Prod.Sci. 83, 211-218.
GEELEN, M., WENSING, T. (2006): Studies on hepatic lipidosis und coinciding health und fertility problems of high-producing dairy cows using the “Utrecht fatty liver model of dairy cows”. A review. Veterinary Quarterly 28, 90-104.
Goff, J. (2006): Major advances in our understunding of nutritional influences on bovine health. . J. Dairy Sci. 89, 1292- 1301.
HERDT, T. H. (2000): Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis und fatty liver. Veterinary Clinics of North America: Food Animal Practice 16, 215-230.
MILLER, R. H., KUHN, M., NORMAN, H. D., WRIGHT, J. R. (2008): Death losses for lactating cows in herds enrolled in dairy herd improvement. J. Dairy Sci. 91, 3710-3715.
GRAVERT, H. O. (1985): Genetic factors controlling feed efficiency. Livestock Prod. Sci. 13, 87-99.
HARRISON, R. O., FORD, S. P., YOUNG, J. W., CONLEY, A. J., FREEMAN, A. E. (1990): Increased milk production versus reproductive and energy status of high producing dairy cows. J. Dairy Sci. 73, 2749-2758.
INGVARTSEN, K. L. (2006): Feeding- and management-related diseases in transition cow. Physiological adaptations around calvin and strategies to reduce feeding-related diseases. Animal Feed Science and Technology 126, 175-213.
INGVARTSEN, K. L., DEWHORST, R. J., FRIGGENS, N. C. (2003): On the relationship between lactational performance and health: is it yield or metabolic imbalance that causes production diseases in dairy cattle? A position paper. Livestock Prod. Sci. 83, 277-330.
IWERSEN, M. (2011): Einfluss von Propylenglykol als Bestandteil einer totalen Mischration während der Frühlaktation auf die Tier- und Stoffwechselgesundheit sowie auf Leistungsparameter von Milchkühen. Dissertation FU Berlin.
MULLIGAN, F.J., DOHERTY, M.I. (2008): Production diseases of the transition cow. Vet. Rec. 176, 3-9.
MARTENS, H., (2007): The Dairy Cow: Physiological Facts and Concerns. Proceedings of the 13th International Conference of Production Diseases in Farm Animals. Ed. M Fürll, Leipzig. P. 26-42 RAUW, W. M., KANIS, E., NORDHUIZEN E. N., GROMMERS, F. J. (1998): Undesirable side effects of selection for high production efficiency in farm animals: a review. Livestock Prod. Sci. 56, 15-33.
SORDILLO, I., CONTRERAS, G., AITKEN, St. (2009): Metabolic factors affecting the inflammatory response of periparturient cows. Animal Health Res. Rev. 10, 53-63 SWALVE, H. (2012): Aktueller Stand der züchterischen Verbesserung von Gesundheit und Funktionalität beim Nutztier. Züchtungskunde 84, 32–38.
Hohe Leistung und lange Nutzungsdauer von Milchkühen:
Ein Praxisbericht
Extrem hohe Milchleistung und erhöhte Abgänge wegen Unfruchtbarkeit oder anderer Ursachen in modernen Kuhherden werden in ursächlichen Zusammenhang gebracht. In dieser Fallstudie wird ein Familienbetrieb mit 145 Kühen und eigener Nachzucht beschrieben, der bewusst von maximaler Laktationsleistung auf hohe Lebensleistung in Kombination mit guter Gesundheit und Lebensdauer umgestellt hat. Als Erfolgsfaktoren werden langjährige Erfahrung, tierfreundliches Management in einem modernen Boxenlaufstall mit Melkroboter, ein spezielles Fütterungskonzept mit hohem Anteil Raufutter aus eigener Produktion, laufende Beobachtung der Tiere und kurzfristige Reaktion auf alle individuelle Gesundheitsprobleme herausgestellt.
Subscribe to our Newsletter
And find out about all the latest industry news.










