
Contact address: v.sommerfeld@uni-hohenheim.de
Dr. Vera Sommerfeld studied Agricultural Biology at the University of Hohenheim in Stuttgart, Germany. Supervised by Prof. Dr. Markus Rodehutscord, she completed her PhD at the Institute of Animal Science at the University of Hohenheim in 2017. She now is a Post-doc mainly focused on poultry nutrition.
Abstract
Broiler chickens show a great potential to degrade phytate, provided that the diet has a low phosphorus (P) and calcium (Ca) content.
Several studies reported that supplementing mineral P and Ca can diminish this potential and provoke the excretion of unused phytate-P.
In this article, two experiments investigating and separating effects of these minerals on phytate degradation are presented.
The first experiment was conducted in an in vitro assay simulating the digestive tract of poultry in three steps to study phytate degradation under standardized conditions.
The second experiment was an in vivo experiment, conducted to study single or interactive effects of P, Ca, and phytase supplementation on phytate degradation in broiler chickens.
It was concluded that the supplementation of either P or Ca can have detrimental effects on the degradation of phytate.
The supplementation of mineral P and Ca to poultry diets should be kept on a minimum to use the full potential of broiler chickens to degrade phytate and thus a maximum of plant-based P.
Keywords:
Broiler chicken, calcium, in vitro, in vivo, phosphorus, phytate degradation.
The endogenous potential of broiler chickens to degrade phytate
Phytate, the salt of myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate), or short InsP6, is the main storage form of phosphorus (P) in plant seeds.
The six phosphate groups adhering to the inositol ring can theoretically be utilized by the animal after stepwise cleavage initiated by phytate degrading enzymes, so-called phytases.
The cleavage of P yields isomers of lower inositol phosphates (InsP) with different degrees of phosphorylation as intermediate products.
In contrast to ruminants, non-ruminant animals like poultry and swine have only a limited endogenous phytase equipment in their digestive tract and it has long been thought that they are not capable to use phytate-bound P.
That is why in practice either mineral P or microbial phytase products are supplemented ‒sometimes even both‒ to meet the P requirements of non-ruminants. During the last years, the view on the non-usable phytate-P has changed, and it is known now that even non-ruminants can utilize some of the phytate-P deriving from the plant components of their feed.
Among non-ruminants, especially broiler chickens show a high potential to degrade phytate and thus use plant-based P. As reviewed by Rodehutscord and Rosenfelder (2016), several studies showed that broilers can degrade up to 62 – 89% phytate when no intrinsic plant phytase or microbial phytase products are included in the diets.
This high degradation is achieved by phytases and other phosphatases produced by the epithelial cells and the microbiota of the intestine. The quantitative contribution of each of the phytase sources is still unknown.
However, a study with gnotobiotic broiler chickens suggests a considerable contribution of epithelial-derived phytases to phytate degradation (42% InsP6 disappearance up to the end of the ileum (prececal) (Sommerfeld et al. 2019).
The high potential of broiler chickens to use phytate-P is usually observed when the diets are low in P and calcium (Ca). It has been shown that phytate-P usage is reduced when feed with standard P and Ca formulations is fed.
Effects of P and Ca supplementation on phytate degradation
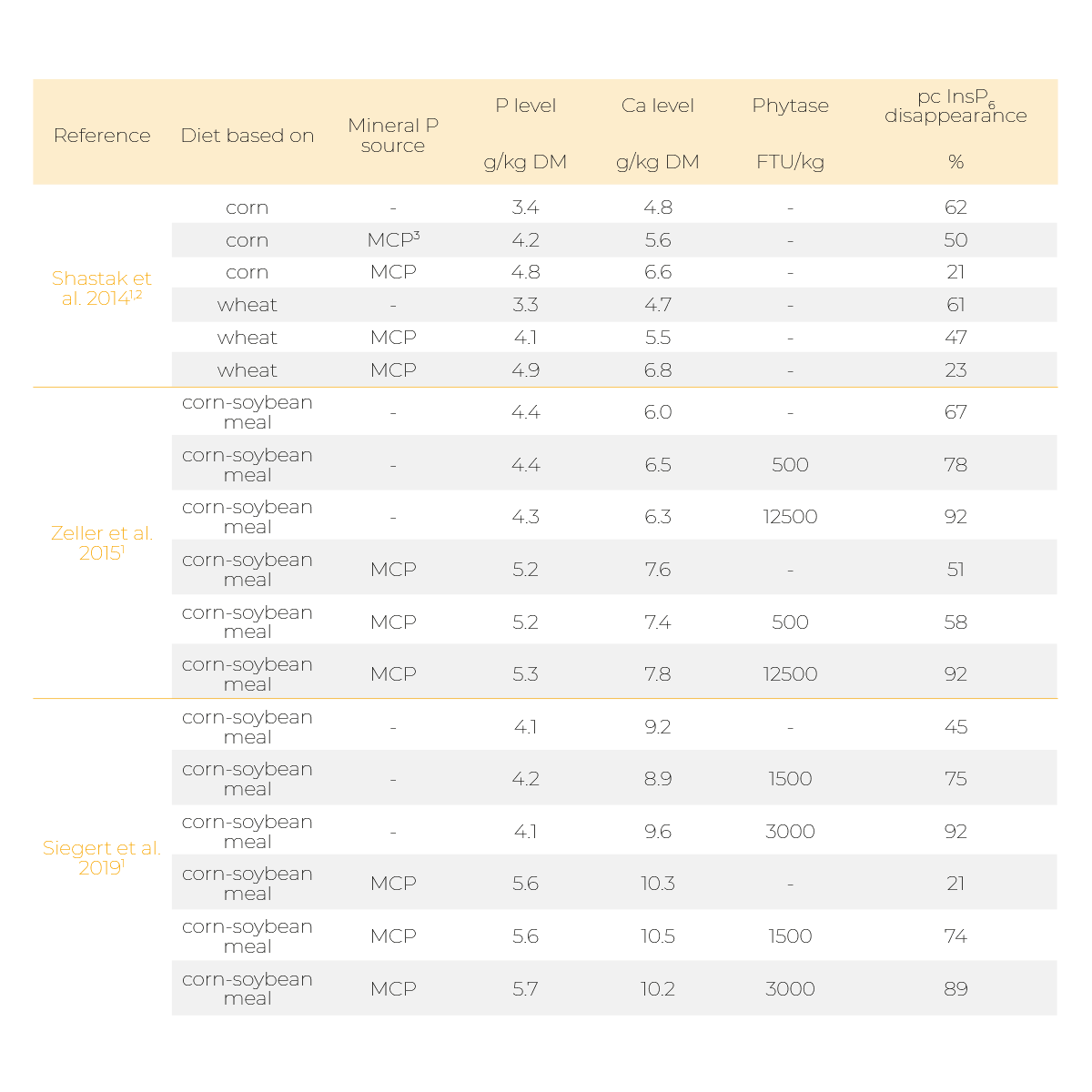
Indeed, it was reported that supplementing mineral P sources like monocalcium phosphate (MCP) or Ca, for example in the form of limestone, can diminish phytate degradation (Table 1 and 2).
2Dietary Ca and P concentration (g/kg DM) calculated assuming 90% dry matter
3MCP, monocalcium phosphate
22 different phytases were used
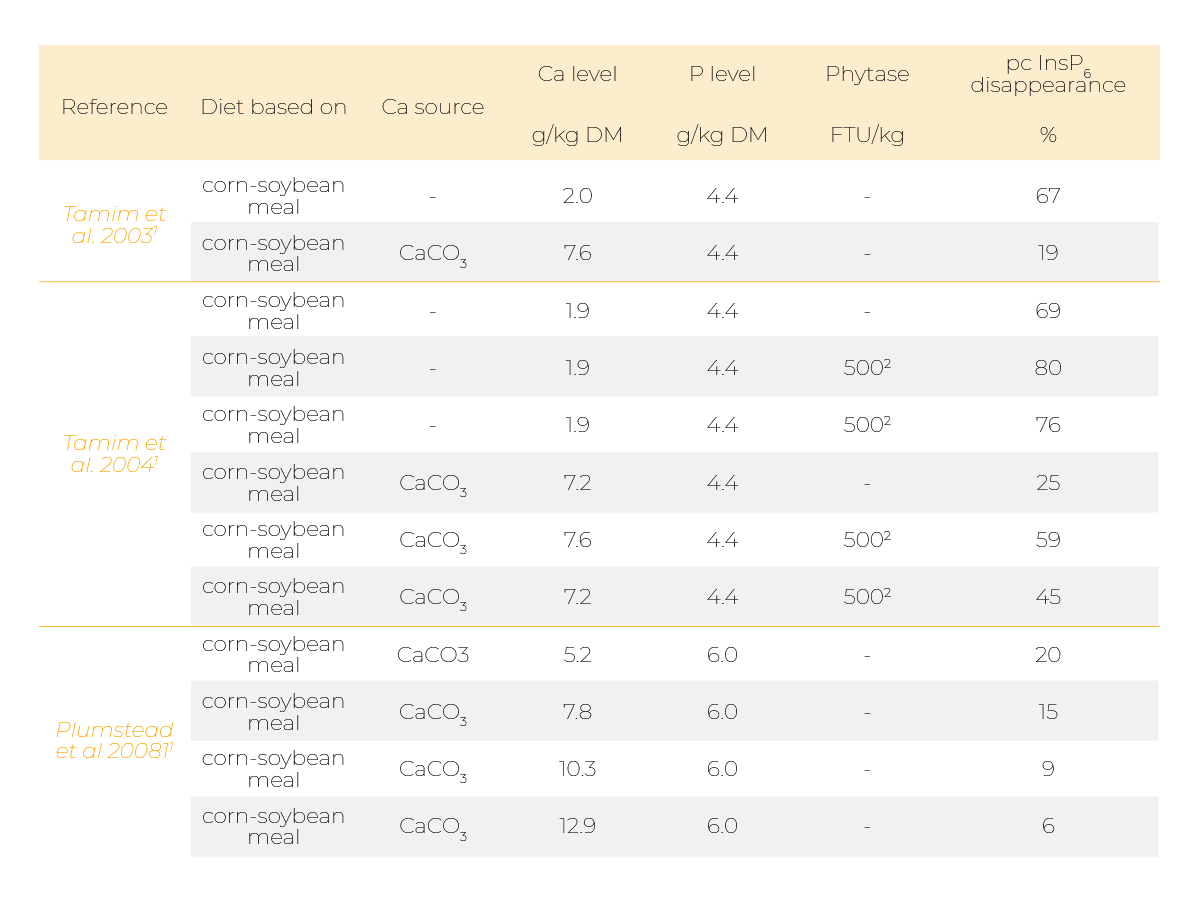
Several studies showed a detrimental effect of supplementing Ca on phytate degradation in broiler chickens (Tamim et al. 2003, 2004; Plumstead et al. 2008; Amerah et al. 2014; Li et al. 2017).
Those effects were usually more pronounced when Ca was supplemented together with P (Manangi et al. 2008; Delezie et al. 2012).
It has also been shown that effects of phytase supplementation were more pronounced when Ca was on a lower level (Tamim et al. 2004; Manangi et al. 2008; Delezie et al. 2012; Amerah et al. 2014).
In broiler studies by Shastak et al. (2014), Zeller et al. (2015), and Siegert et al. (2019), a decrease in prececal InsP6 disappearance was observed when a mineral P source was supplemented to a low P/low Ca diet in the absence of a microbial phytase (Table 1).
The diminishing effect of P and Ca supplementation was repealed by a very high phytase dosage (Zeller et al. 2015 and Siegert et al. 2019).
However, the diminishing effects could still be observed in a slower degradation of lower phosphorylated InsP isomers. In all three studies, P and Ca were supplemented simultaneously which made it impossible to distinguish whether the observed effects derived from the supplemented P, Ca or the combined supplementation.
To address this question, several experiments were conducted in the Animal Nutrition group of the Institute of Animal Science in Hohenheim.
With the aim to implement a fast and cost-effective investigation method and to reduce animal experiments, an in vitro assay was established in our laboratory to simulate the poultry’s digestive tract and to work on this research question.
In vitro assay and its application to study P and Ca effects on phytate degradation
In vitro assays are valuable tools to obtain fast and reproducible results. Animal trials are more time consuming, expensive and need a considerable number of animals.
A three-step in vitro assay simulating the crop, stomach and small intestine of poultry as described by Zyla et al. (1995) was used as a template. It was modified for our purposes to study the degradation of phytate and the formation of lower phosphorylated InsP isomers.
In each step, a certain retention time, temperature, water content, pH and digestive enzymes are applied or supplemented (see Figure 1).
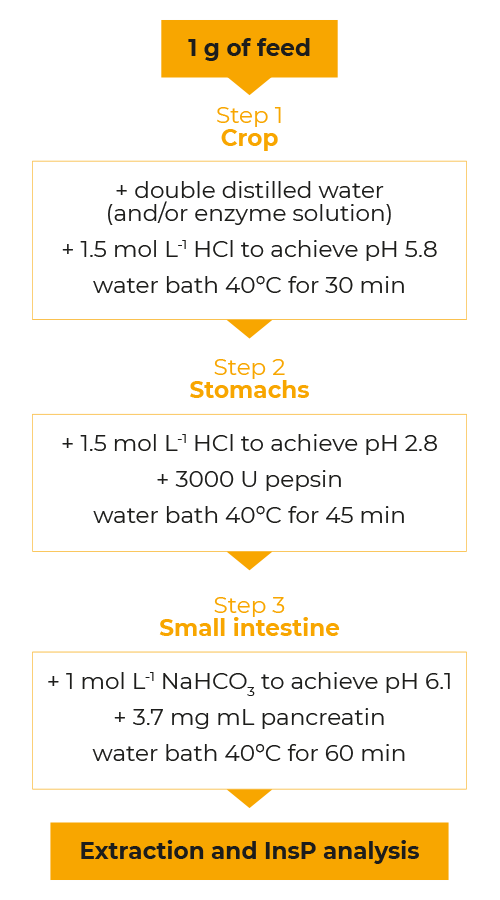
A mixed broiler feed based on corn and soybean meal low in P and Ca was used as phytate matrix. This contrasts classical in vitro assays for phytase characterization in which purified sodium phytate is usually used as a matrix.
However, it was shown that phytate degradation in general, and phytase efficacy in particular, depends on the phytate matrix. Therefore, purified sodium phytate is considered not to be the best choice for phytase efficacy studies.
In an experiment that is described in detail in Sommerfeld et al. (2017), first attempts have been made to distinguish between single or interactive effects of P and Ca supplementation in a low P and Ca corn-soybean meal-based broiler feed (herein referred to as Basal).
Different P and Ca levels were achieved by supplementing NaH2PO4 (+P and +high P) or limestone (+Ca and +high Ca), or both (+CaP). Three phytase concentrations were applied (0, 150 and 300 FTU/kg). After incubation of the respective feed mixtures, the InsP concentrations in the feed slurries were analyzed.
It was shown that the mineral supplements had no effect when no microbial phytase was supplemented (Figure 2). This occurred since phytate degradation was in general very low without supplemented phytase.
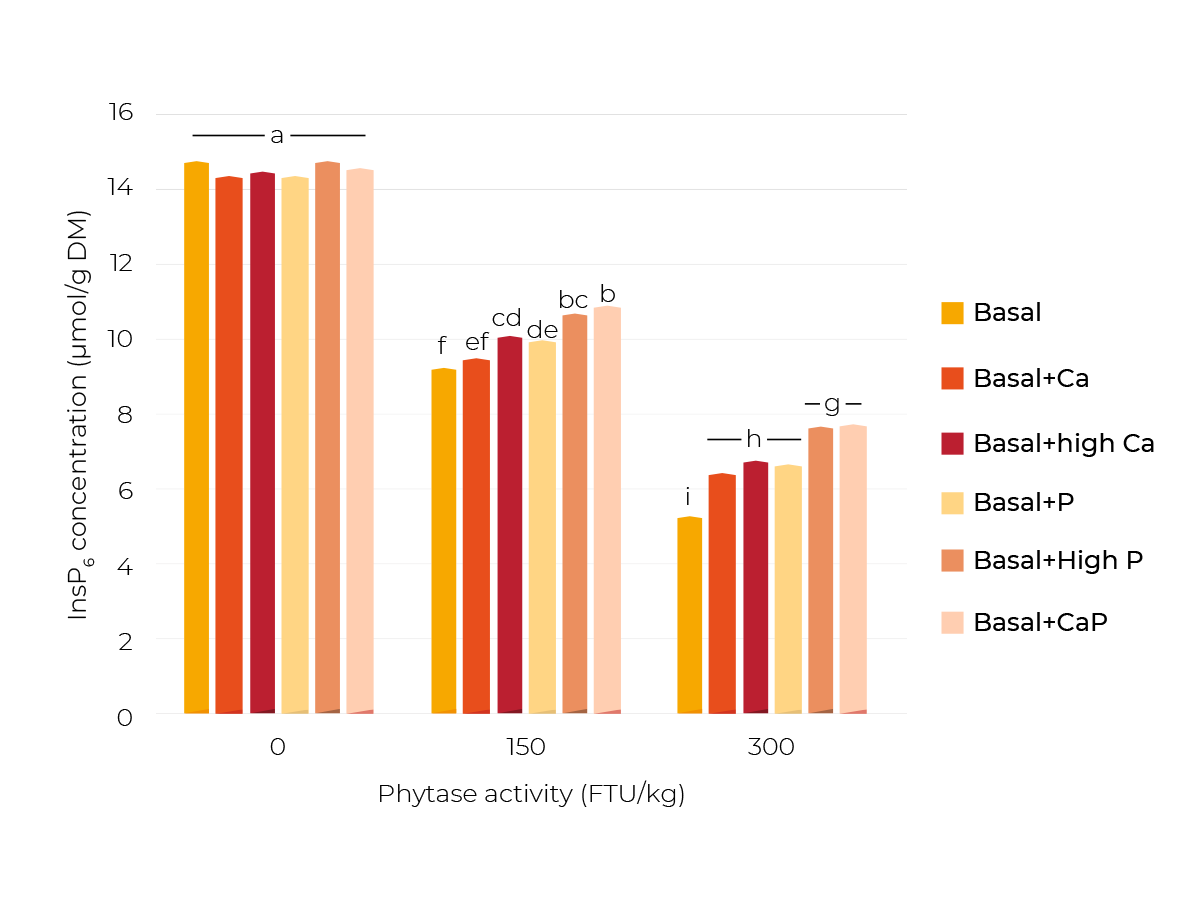
This was expected because the phytase activity of corn and soybean is negligible.
After adding 150 FTU phytase/kg, there was a significant higher InsP6 concentration in +highCa and +P than in the Basal diet.
With +highP or +CaP, the highest concentration, thus lowest degradation of InsP6 was achieved. With 300 FTU phytase/kg, these effects were even clearer.
These results show that mineral supplements can have a diminishing effect on phytate degradation. The diminishing effects of the supplemented P and Ca might be explained by a direct inhibitory effect of P on the added phytase and the formation of Ca-phytate complexes.
Results further demonstrate that the in vitro assay reacts in a plausible manner to different supplements. Phytases can be tested prior to an animal trial in an environment similar to the poultry’s digestive tract.
This makes it a suitable, standardized tool to preselect treatments for animal trials. This practice avoids unnecessary treatments and reduces the number of test animals.
However, the absolute values of InsP concentrations after incubation do not directly reflect in vivo results. As in any in vitro assay, many conditions prevailing in poultry’s digestive tract cannot be simulated.
Animal related factors like endogenous enzymes from epithelial cells or the microbiota are not considered.
Despite the advantages of this in vitro assay, it is still necessary to evaluate dietary effects in the animal model. For that reason, an in vivo trial was conducted to separate P and Ca effects on InsP degradation in broiler chicken.
In vivo trial to study P and Ca effects on phytate degradation
In this trial, which is described in detail in Sommerfeld et al. 2018, broilers were fed 8 diets based on corn and soybean meal from day 15 to 27. The diets comprised 2 P levels (P-, 4.1 g P/kg DM and P+, 6.9 g P/kg DM), 2 Ca levels (Ca-, 6.2 g Ca/kg DM and Ca+, 10.3 g Ca/kg DM) and 2 phytase levels (0 and 1500 FTU phytase/kg).
All single and interactive effects among these supplements were tested.
The prececal InsP6 disappearance in the absence of microbial phytase (56%) was decreased by the supplementation of P (40%) and even more when P and Ca were added simultaneously (21%; Table 3).
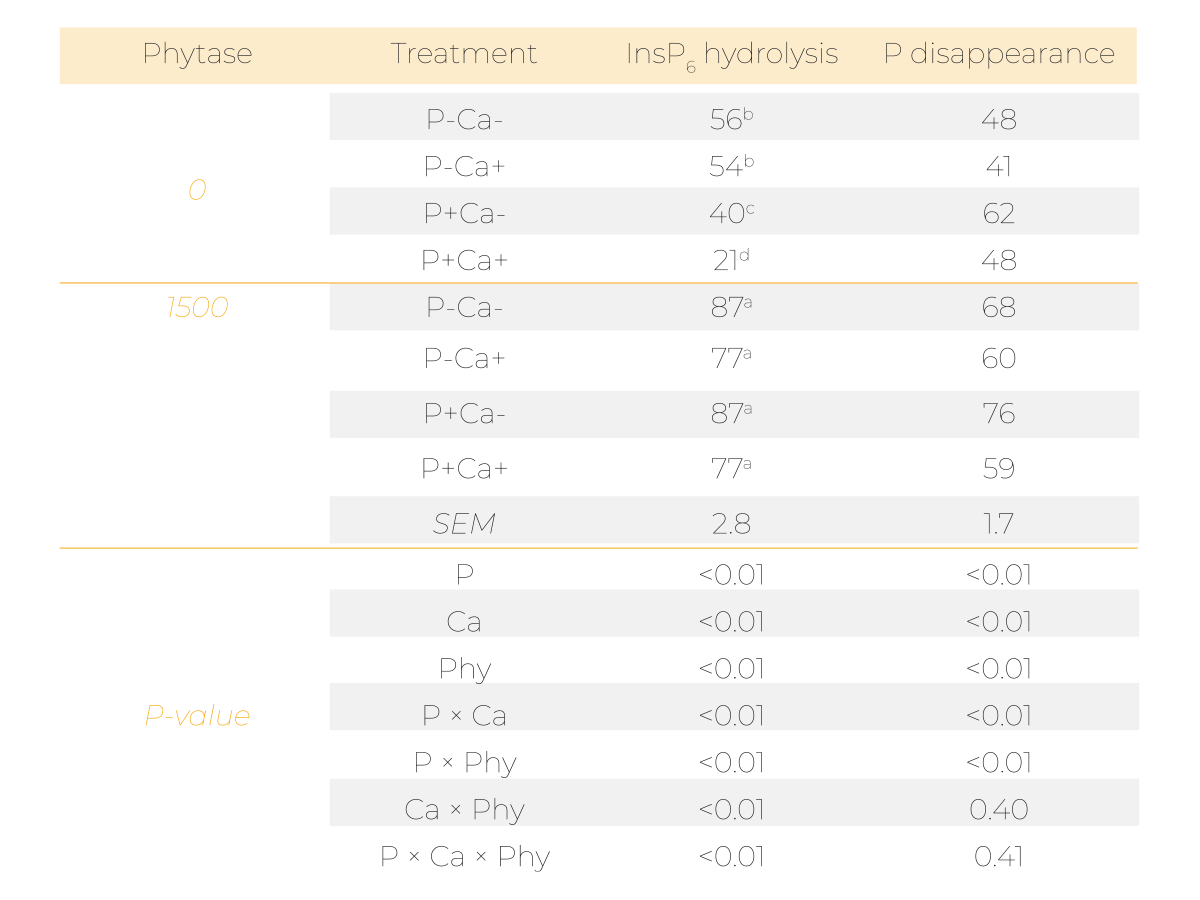
When phytase was supplemented, mineral supplements had no diminishing effect (77-87%) on prececal InsP6 disappearance. However, the mineral supplements had clear effects on the degradation of lower phosphorylated InsP (Figure 3).
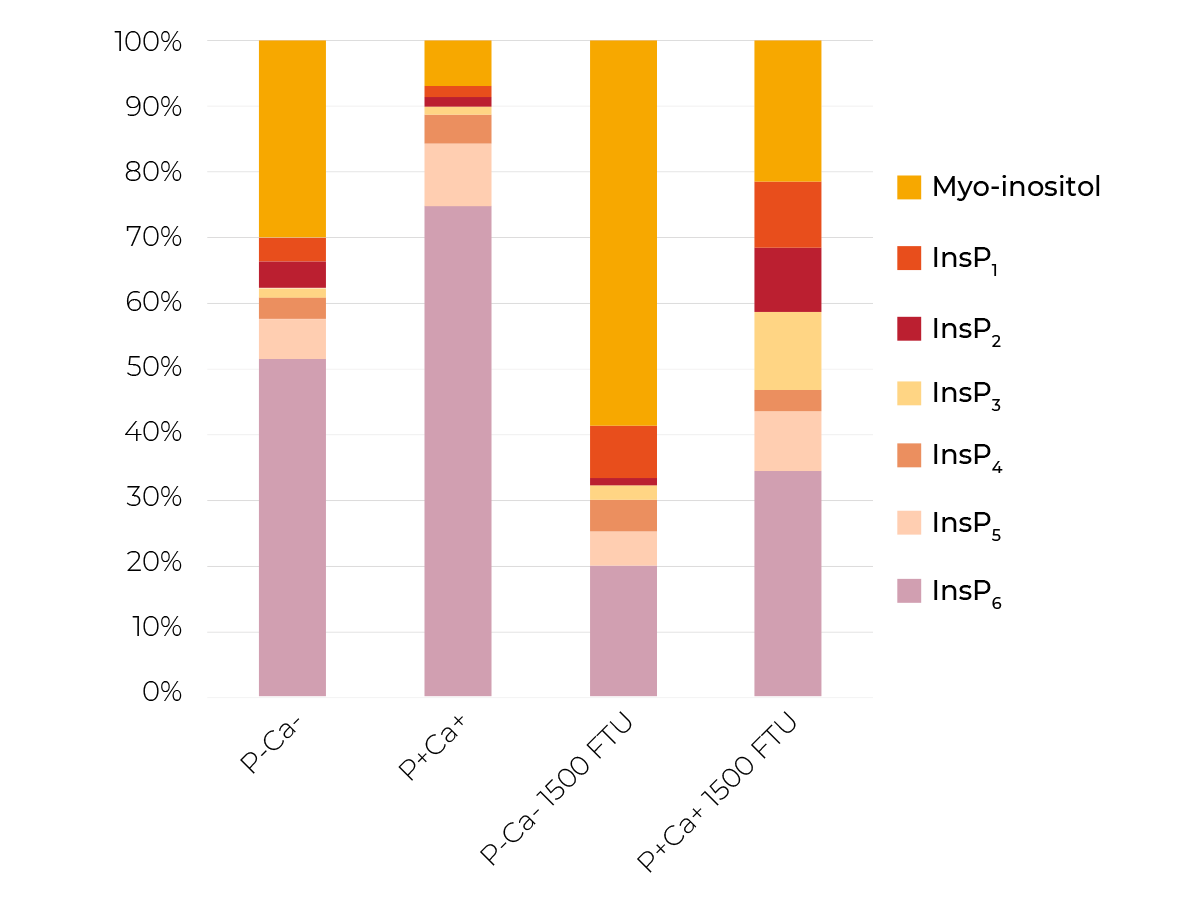
While the InsP6 disappearance did not differ significantly between the treatments P-Ca- and P+Ca+ in the presence of phytase, the relative proportion of the single InsP and the end product myo-inositol (when all P groups are released) differed markedly between those two treatments. P-Ca- had a lower proportion of InsP6 and InsP1-5 and consequently a higher proportion of myo-inositol than P+Ca+.
Possible causes include a diminishing effect of mineral supplements especially on the activity of endogenous epithelial and microbiota-derived phosphatases.
This circumstance becomes relevant in the context of efficient P usage and avoidance of unnecessary P excretion. Even if InsP6 has disappeared, a considerable amount of P can still be attached to lower phosphorylated InsP, remain unabsorbed and, hence, is subject to unfavourable effects of poultry farming. This is reflected in the prececal P disappearance that was significantly reduced by Ca supplementation (Table 3), supporting the findings regarding InsP degradation.
Conclusions
The results of these two trials ‒in vitro and in vivo‒ clearly demonstrate a diminishing effect of P, especially in combination with Ca supplementation on intestinal phytate degradation. Possible causes include affected endogenous phosphatases and formation of mineral-phytate complexes.
Several studies from our group (Künzel et al. 2019, Shastak et al. 2014, Siegert et al. 2019, Sommerfeld et al. 2018, Sommerfeld et al. 2019, Zeller et al. 2015) confirm these results and show that even low amounts of supplemented P are detrimental for phytate degradation.
Therefore, a certain amount of potentially usable plant-based P will be left unused and excreted. As not only InsP6, but also lower phosphorylated InsP are affected by mineral supplements, this can have an impact on 1) the utilisation of plant-based phytate-P for the P requirement of the bird and 2) the excretion of unused phytate-P, leading to environmental issues.
In terms of aiming for a sustainable usage of mineral P and the reduction of excreted P due to environmental aspects, supplementation of P and Ca should be kept on a level where the full potential of broiler chickens to degrade phytate can be used without negatively affecting bird health and growth.
References
Amerah, A. M., P. W. Plumstead, L. P. Barnard, and A. Kumar. 2014. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult. Sci. 93:906–915.Delezie, E., L. Maertens, and G. Huyghebaert. 2012. Consequences of phosphorus interactions with calcium, phytase, and cholecalciferol on zootechnical performance and mineral retention in broiler chickens. Poult. Sci. 91:2523–2531.
Künzel, S., D. Borda-Molina, R. Kraft, V. Sommerfeld, I. Kühn, A. Camarinha-Silva, and M. Rodehutscord. 2019. Impact of coccidiostat and phytase supplementation on gut microbiota composition and phytate degradation in broiler chickens. Animal Microbiome 2:1.
Li, W., R. Angel, S.-W. Kim, K. Brady, S. Yu, and P. W. Plumstead. 2017. Impacts of dietary calcium, phytate, and phytase on inositol hexakisphosphate degradation and inositol phosphate release in different segments of digestive tract of broilers. Poult. Sci. 96:3626-3637.
Manangi, M. K., and C. N. Coon. 2008. Phytate phosphorus hydrolysis in broilers in response to dietary phytase, calcium, and phosphorus concentrations. Poult. Sci. 87:1577–1586.
Plumstead, P. W., A. B. Leytem, R. O. Maguire, J. W. Spears, P. Kwanyuen, and J. Brake. 2008. Interaction of calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult. Sci. 87:449–458.
Rodehutscord, M., and P. Rosenfelder. 2016. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. Pages 15-32 in Phytate Destruction.
C. L. Walk, I. Kühn, H. H. Stein, M. T. Kidd, and M. Rodehutscord, eds. Wageningen Academic Publishers, Wageningen.
Shastak, Y., E. Zeller, M. Witzig, M. Schollenberger, and M. Rodehutscord. 2014. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 93:2548-2559.
Siegert, W., T. Zuber, V. Sommerfeld, J. Krieg, D. Feuerstein, U. Kurrle, and M. Rodehutscord. 2019. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult. Sci. 98:5700-5713.
Sommerfeld, V. 2018. In vivo and in vitro studies of degradation of inositol phosphates in the digestive tract of broiler chickens. Dissertation. Universität Hohenheim, Stuttgart-Hohenheim. Institut für Nutztierwissenschaften.
Sommerfeld, V., M. Schollenberger, L. Hemberle, and M. Rodehutscord. 2017. Modification and application of an in vitro assay to examine inositol phosphate degradation in the digestive tract of poultry. J. Sci. Food Agric. 97:4219-4226.
Sommerfeld, V., M. Schollenberger, I. Kühn, and M. Rodehutscord. 2018. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 97:1177-1188.
Sommerfeld, V., A. G. Van Kessel, H. L. Classen, M. Schollenberger, I. Kühn, and M. Rodehutscord. 2019. Phytate degradation in gnotobiotic broiler chickens and effects of dietary supplements of phosphorus, calcium, and phytase. Poult. Sci. 98:5562-5570.
Tamim, N. M., and R. Angel. 2003. Phytate phosphorus hydrolysis as influenced by dietary calcium and micro-mineral source in broiler diets. J. Agric. Food Chem. 51:4687-4693.
Tamim, N. M., R. Angel, and M. Christman. 2004. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 83:1358–1367.
Zeller, E., M. Schollenberger, M. Witzig, Y. Shastak, I. Kühn, L. E. Hoelzle, and M. Rodehutscord. 2015. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 94:1018-1029.
Zyla, K., D. R. Ledoux, A. Garcia, and T. L. Veum. 1995. An in vitro procedure for studying enzymic dephosphorylation of phytate in maize–soyabean feeds for turkey poults. Br. J. Nutr. 74:3-17.







