Summary
Acidification of drinking water and feed has been used for years in poultry to control Salmonella. For
drinking water and powder form feed supplementation, the choice of the acids used is dependent on
its ability to kill bacteria. Currently also coated or impregnated acid products are on the market, aiming
to bring the acids further down in the gastro-intestinal tract. It was shown that supplementation of
acetic acid coated products to the feed increases colonization of the chicken gut by Salmonella, and
that coated propionic and butyric acid products decreases colonization of the chicken intestinal tract.
Moreover, it was shown that coated butyric acid decreased faecal shedding in a seeder model in
broilers throughout the rearing period up to slaughter age. This can be linked with effects of the acids
on Salmonella virulence, since exposure of Salmonella to low concentrations of acetic acid increases
invasion of Salmonella in intestinal epithelial cells, while propionic and butyric acid decrease invasion at non growth inhibitory concentrations. This is due to changes in virulence gene expression of
the genes of the Salmonella Pathogenicity Island I, necessary for invasion. Currently the approach
of increasing butyric acid producing gut microbiota is under research in different institutes. Increasing
the concentration of butyrate producers in poultry caeca using nutritional strategies and addition of
pro- or prebiotics would be an efficient way to combat Salmonella infections. Indeed, these bacteria
produce butyric acid at the site where Salmonella is predominantly colonizing and invading, i.e. the
caeca. Furthermore, after years of adding feed additives to poultry feed in an empirical way, the
approach could finally be more rational, aiming at an increase in the butyrate producing flora in chicken
caeca.
LOHMANN Information is a recognized, scientific publication in English language, focused on life
science, human nutrition, animal and poultry breeding, production, nutrition, health and welfare.
LOHMANN BREEDERS has a history of supporting this independent publication in its distribution.
The content is the responsibility of the editor Prof Werner Bessei (Professor em. Farm Animal Ethology and Small Animal Science) and is coordinated with the authors.
We have selected an interesting article for you.
We wish you an interesting reading experience.
LOHMANN BREEDERS has a history of supporting this independent publication in its distribution.
The content is the responsibility of the editor Prof Werner Bessei (Professor em. Farm Animal Ethology and Small Animal Science) and is coordinated with the authors.
We have selected an interesting article for you.
We wish you an interesting reading experience.
Introduction
Poultry meat and eggs are important food products and the poultry-related industry is an economically important component of the agro-industry in the EU. The industry however is under pressure and faces many challenges. Most importantly, there is a demand for eggs and meat of high nutritional value and free of microbiological and chemical hazards. Unfortunately poultry eggs and meat are one of the major sources of food borne bacterial infections in humans. In this respect, Salmonella (mainly Salmonella Enteritidis) and Campylobacter are of particular importance, since these pathogens can colonize the chicken host without causing discernible illness in the infected chickens.In Europe and the USA, Salmonella Enteritidis has become by far the most important egg-associated pathogen resulting in a pandemic of non-typhoidal salmonellosis in humans. Despite the recent decrease in human Salmonella Enteritidis infections due to the introduction of control measures in layer flocks, Salmonella still is one of the most important bacterial foodborne infections in the world. Serotypes other than Enteritidis are not decreasing in prevalence to the same magnitude as Enteritidis.
While some serotypes, such as Derby and Typhimurium, can be traced back to porcine meat, other serotypes, such as Virchow, Infantis, Hadar and again Typhimurium, can be traced back to poultry meat. Since poultry meat will, due to EU legislation, not be brought as fresh poultry meat on the market from 12/12/2010 if Salmonella is detected, the broiler industry needs to take measures to decrease the colonization of the animals and their environment.
These include pre-harvest, harvest and post-harvest measures. All of these are equally important and each type of measure has a more or less important effect on reducing the Salmonella incidence, but no measure is successful on its own. Harvest measures are essentially hygienic measures during catching and transport, while post-harvest measures include both hygienic measures and the application of decontaminating treatments on the meat.
However, all carcass disinfectants are prohibited in the European Union at present, thus decontamination is not an option. Therefore, prevention and monitoring/eradication during the live phase (pre-harvest) are of great importance in Europe. Pre-harvest prevention and control measures start in dedicated feed mills and on the grandparent farms of primary breeders (MacLeod, 2002) and include preventive hygienic measures as well as physical and chemical decontamination treatments of feed, drinking water and the environment of the birds down to the commercial farms of broiler growers and egg producers. Since antibiotics are not the method of choice to control Salmonella and since vaccination cannot be applied in broilers due to the short life span of the animals, the use of feed additives is more and more accepted as a valuable way to combat bacterial gut infections.
Currently however there are numerous products on the market and it is not easy for poultry producers to objectively choose products with proven activity against Salmonella, since most of the products are empirically produced.
The use of organic acids against Salmonella
A) Effects of short-chain fatty acids on survival and virulence of Salmonella in vitro.Medium chain fatty acids (C6 to C12; caproic, caprylic, capric and lauric acid) appear to be much more effective against Salmonella than the short-chain fatty acids (formic, acetic, propionic and butyric acid). As little as 25mM C6 to C10 acids were bacteriostatic to a Salmonella Enteritidis strain, while the same strain tolerated 100mM of short-chain fatty acids (Van Immerseel et al., 2003, 2004a). When Salmonella Enteritidis and Typhimurium were incubated with low concentrations of monocaprin (5 mM) Preventing Salmonella infections by rationally designed feed additives: Vol. 42 (1), April 2007, Page 10 that had been combined with an emulsifier, the bacteria did not survive (Thormar et al., 2006). In general, these data indicate that medium chain fatty acids have the greatest antibacterial activity against Salmonella, but large scale studies are lacking.
Salmonella is an opportunistic intracellular pathogen that has an elaborate set of virulence genes. These genes enable the bacterium to adapt to the environment and move between various microniches within a host. An early step in the pathogenesis of Salmonella is the penetration of intestinal epithelium.
This activity is promoted by invasion genes that are located on a pathogenicity island (SPI-1), but several pathogenicity islands are required for full virulence. SPI-1 has genes encoding regulatory proteins, structural components of a needle complex and additional effector proteins. Bacterial effector proteins facilitate the entry of Salmonella into the cytosol of epithelial cells, by inducing actin rearrangements that lead to uptake of the bacteria. SPI-1 is in turn activated by HilA, and this latter protein is environmentally regulated. When Salmonella Typhimurium was pre-incubated in growth media supplemented with various concentrations of butyrate and propionate, epithelial cell invasion was suppressed (Van Immerseel et al., 2003). However, if the bacteria were preincubated in media supplemented with acetate invasion was still observed.
Similar results were obtained with Salmonella Enteritidis when primary caecal epithelial cells of the chicken were employed. The effects of organic acids on epithelial invasion can be explained by changes in SPI-1 expression. When HilA and invF (major activators of SPI-1) expression was measured in S. Typhimurium after exposure to acetate, butyrate and propionate at pH 6, exposure of the bacteria to acetate increased the expression of these genes, but similar effects were not observed with propionate or butyrate (Durant et al., 2000).
More recently, it was shown that butyrate and propionate, but not acetate led to a decrease in hilA, invF and sipC expression (Lawhon et al., 2005) DNA microarrays of both Salmonella Typhimurium and Salmonella. Enteritidis indicated that low doses of butyric acid downregulated SPI-1, but it did not alter metabolic gene expression (Gantois et al., 2006). The primary target of butyrate in the bacterial cell is still unknown but butyrate could interfere with HilA dependent regulation of SPI1 by altering the regulation of hilD transcription. These data indicate that short-chain fatty acids can regulate the invasive phenotype of Salmonella.
B) Effects of short-chain fatty acids in Salmonella control in vivo
The use of acidic compounds to control Salmonella first appeared in the late 1960’s, and mainly focused on decontamination of bone meal. Evaluation of the efficacy of 32 different acid preparations to decontaminate bone meal showed that low molecular weight volatile fatty acids were the most promising (Khan and Katamay, 1969). These results were a basis for the development of non-toxic, naturally occurring acidic compounds to control Salmonella. More than 35 years later, it is clear that their thoughts were prophetic. These acids have been added to feed, drinking water, and other matrices, in order to prevent Salmonella colonization of animal tissue and transmission through the food chain.
Poultry feed is a major source for Salmonella introduction to the farm. When chickens are given artificially contaminated feed, the gut is colonized and Salmonella are shed into the environment. The original concept of incorporating acids into feed was based on the notion that the acids would decontaminate the feed itself and prevent Salmonella uptake by the chickens.
When Iba and Berchieri (1995) inoculated feed with high doses of a S. Typhimurium strain, a commercial mixture of formic and propionic acid decreased the viability more than 1000-fold over 7 days. When broiler chicks were given the acid treated feed that had been mixed with either Salmonella Enteritidis, Typhimurium or Agona, the caecal Salmonella numbers were 7 logs lower than control animals at day 5 (102 versus 109 cfu/g). Mixtures of formic and propionic acid were also effective when feed was artificially inoculated with low doses of S. kedougou, and the decrease was most obvious after several weeks of storage. In a large scale study, the number of Salmonella positive breeder feed samples decreased from 4.1 to 1.1% after the feed was supplemented with 0.5% formic acid. The Preventing Salmonella infections by rationally designed feed additives: Vol. 42 (1), April 2007, Page 11 antibacterial activities of organic acids were dependent on the temperature and moisture. Since the water content of poultry feed is generally low, the action of the acids is not always optimal, and it is not clear whether effects of the acids in the feed itself or effects of the acids in the gastrointestinal tract of the animals were the major reason of protection.
In the 1980’s, it became clear that the acid concentrations were also increased in the crop, and this antibacterial action could aid in controlling infection caused by horizontal transmission. Indeed, when the acid treated feed is eaten by the chickens, it is both warmed and moistened and the activity of the short chain fatty acids should increase. It appears that supplemental acids are most apt to affect in the crop and gizzard rather than in the intestine. This point is illustrated in a study of Thompson and Hinton (1997), who fed laying hens a feed supplemented with a commercial mixture of formic and propionic acid. In these animals, pH values of the crop, gizzard, jejunum, caecum and colon were not altered relative to control animals, but formic and propionic acid concentrations in crop and gizzard were significantly increased. At the same time, the lactic acid concentration in the crop decreased significantly, suggesting that lactobacilli were either inhibited or killed.
Later in the 1980’s, many studies examined the effects of supplementing acids on Salmonella colonization of chicken tissues. Mainly the action of formic and propionic acids was tested. In a smallscale field trial, formic acid controlled shedding and caecal colonization by Salmonella serovars in naturally infected the animals. Indeed, 50% of all control animals had Salmonella positive cloacal swabs and caecal content samples, but Salmonella could not be detected in animals that consumed significant concentrations of formic acid (Hinton et al., 1985).
In a 3 year study, the cumulative number of infections of newly hatched chicks with Salmonella decreased after breeder stocks were given formic acid treated feed (Humphrey and Lanning, 1988). Breeders that received acidified feed shed lower numbers of Salmonella in the litter (4.3 versus 1.4%), hatchery waste (15.3 versus 1.2%) and insert paper samples (4.6 versus 1.4%). These decreases were evident from the moment the breeders received acidified feed and illustrate the effects of vertical transmission.
The most striking proof of the efficacy of formic and propionic acid as feed additives to control Salmonella was given by Hinton and Linton (1988). In three independent experiments, only natural infections were monitored. Formic and acetic acid supplemented feed, given from the day of hatch, decreased the number of positive faeces and caecal content samples dramatically. The control groups had 25, 27 and 60% Salmonella positive faecal samples, but the treatment groups were 3, 0 and 0%. When the formic acid treated feed was given at later age (16 or 32 days), no differences were detected between control and treated groups. This illustrates that preventing initial colonization of Salmonella is most important. Once an infection is established, it is very difficult to counteract by using acid treated feed, at least in the same production round.
Recently, researchers have attempted to transport the organic acids further down in the gastrointestinal tract by micro-encapsulation, which should prevent absorption of the acids in the upper tract and ensure release further down in the gastro-intestinal tract. Van Immerseel et al. (2004b) examined the effect of microbeads containing formic, acetic, propionic and butyric acid on colonization of S. Enteritidis in caeca, liver and spleen. Animals were infected (day 5 post-hatch) with 5 x 103 cfu S. Enteritidis and samples were taken 3 days post-infection. Cecal colonization was significantly increased when acetic acid was added to the feed, but decreased when butyric acid was added (Table 1). Internal organ colonization was increased if either formic or acetic acid were added to the feed, and this result is consistent with the idea that acids can enhance the virulence of Salmonella.
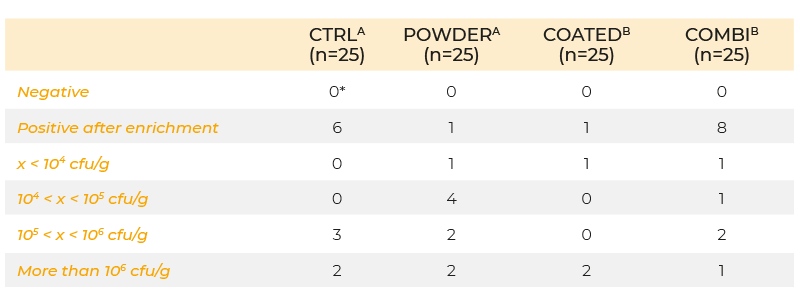
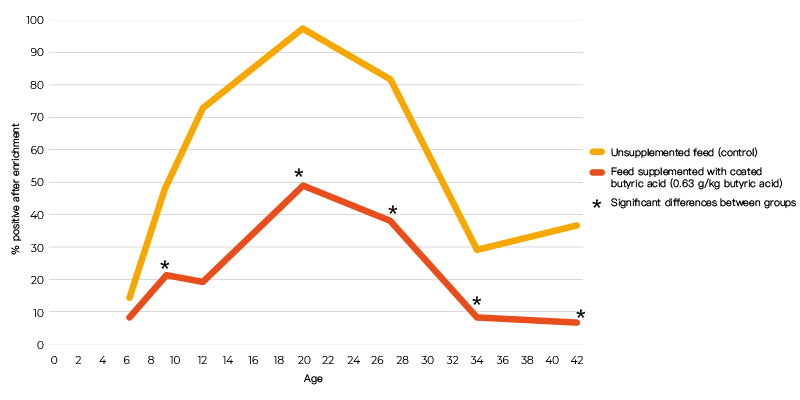
When powder and coated butyric acid additives (0.63 g/kg butyric acid) were compared using the same infection protocol, the coated form decreased colonization of the caeca, but the powdered form did not (Table 2). The inability of the powered form to give a positive response may have been due to the short time interval between infection and sampling. In an infection study using a seeder model in which 10 broilers were infected at day 5 post-hatch with 105 cfu S. Enteritidis and housed together with 40 non-inoculated broilers, 0.63 g/kg coated butyric acid in the feed significantly reduced shedding of S. Enteritidis in broilers until slaughter age (Van Immerseel et al., 2005, (Figure 1)). The effect of the acids on other members of the microbial community was not determined.
Table 1. Colonization of the caeca at day 8 of life (inoculation with 103 cfu S. Enteritidis
76Sa88 on day 5 and 6) in chickens fed a diet supplemented with formic, acetic,
propionic and butyric acid or no feed additives.
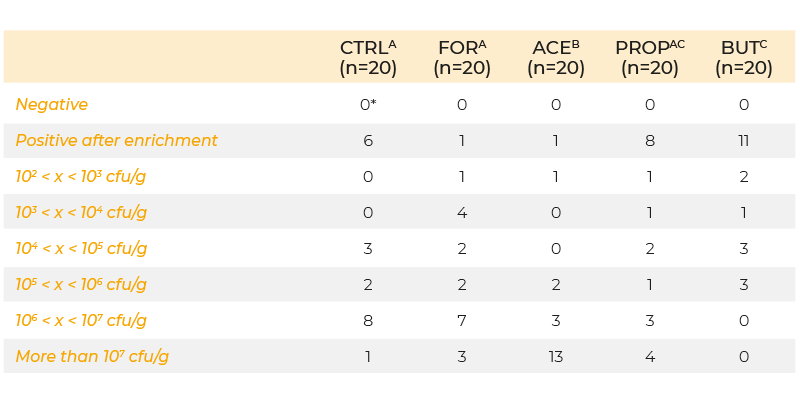
Table 2. Colonization of the caeca at day 8 of life (inoculation with 106 cfu S. Enteritidis
76Sa88 on day 5) in chickens fed a diet supplemented with butyric acid in powder
form, coated form, a combination of half doses of powder and coated form (COMBI),
or no feed additives (CTRL). Concentration of the active product butyric acid was
0.63 g/kg feed in each group.
Increasing caecal butyrate concentration to control Salmonella: use of pre- and probiotic approaches?
Feed and drinking water sanitation, and the addition of acids to the crop appears to prevent pathogen colonization in the live animals, but the type of acid and its concentration can be very important. Salmonella colonization of the caeca and internal organs is not always affected by these treatments, especially if the infection pressure is high. Acids from feed or drinking water are not effective further down in the intestinal tract because Salmonella colonization is mainly in the caeca. Because the caecum is the main fermentation site, the concentrations of short-chain fatty acids are already higher there than in other intestinal segments. It has already been shown in various animal species that.
Figure 1. Number of positive cloacal swabs over time (age) following infection of 10 chicks
with 105 cfu S. Enteritidis 76Sa88 at day 5 post-hatch and housing them with 40
non-inoculated broilers.
Salmonella colonization of the gut is decreased when the bifidobacterial population is increased, either by administration of bifidobacteria as probiotic strains, or by addition of certain types of oligosaccharides that stimulate proliferation of these bacteria in the gut (Asahara et al., 2001; Buddington et al., 2002; Bovee-Oudenhoven et al., 2003; Silva et al., 2004; Thitaram et al., 2005).
Increases in lactic acid bacterial counts in the gut are correlated with increases in butyric acid concentrations, and Salmonella colonization is decreased when butyric acid levels in the gut are increased. Bifidobacteria increase butyric acid concentrations, but these bacteria do not produce butyric acid themselves. Lactic acid bacteria, such as lactobacilli and bifidobacteria, stimulate proliferation of butyric acid producing bacteria. This mechanism is called cross-feeding.
It has been shown that lactic acid, produced in vitro by Bifidobacterium adolescentis with starch as sole carbon source, is used by Anaerostipes caccae and Eubacterium hallii (in co-culture) for the production of high concentrations of butyric acid. Another approach would be a direct stimulation of butyric acid producing bacteria. In human gut samples, butyric acid producers are anaerobic bacteria belonging to the phylogenetic Clostridium clusters IV and XIVa, and species related to Roseburia, Eubacterium, Faecalibacterium and Coprococcus can also produce butyrate (Pryde et al., 2002; Duncan et al., 2004). Many of the butyrate producing microbiota that are identified are net consumers of acetate. Random cloning and sequencing of 16SrDNA sequences isolated from chicken caeca revealed more than 85% of the clones belonging to eubacteria and clostridia (Bjerrum et al., 2006). Approximately 10% of the clones had high similarity with Faecalibacterium prausnitzii, a species that produces butyric acid in the human gut.
When butyric acid producing bacteria from poultry caeca were isolated (producing more than 10mM in culture) in the author’s lab, 16SrDNA sequence analysis showed that these isolates were rather closely related to the butyrate producing species described for humans. The isolates consumed acetate and produced butyrate, and are most likely also cross-fed by lactate. These data can open new opportunities for Salmonella control. Indeed, increasing the concentration of these butyrate producers in poultry caeca using nutritional strategies and addition of pro- or prebiotics would be an efficient way to combat Salmonella infections.
Chances of success are increased, since these bacteria produce butyric acid at the site where Salmonella is predominantly colonizing and invading. Furthermore, after years of adding feed additives to poultry feed in an empirical way, the approach could finally be more rational, aiming at an increase in the butyrate producing flora in chicken caeca, finally providing a way to measure the action of the additives. The criteria for Salmonella control thus would be: do the additives increase the amount and distribution of butyrate producing bacteria in chicken caeca?
Currently tests are being developed to quantify the presence of the key enzyme involved in butyrate synthesis in the anaerobic butyrate producing flora at the DNA level, in order to produce a simple and reliable test to evaluate the effect of additives and Salmonella control, even without performing large scale infection trials. This would enable us to rationally design feed additives in the future.
Asahara, T., Nomoto, K., Shimizu, K., Watanuki, M. and tanaka, R. (2001). Increased resistance of mice to Salmonella enterica serovar Typhimurium infection by symbiotic administration of Bifidobacteria and transgalactosylated oligosaccharides. Journal of Applied Microbiology 91, 985-996.
Bjerrum, L., Engberg, R.M., Leser, T.D., Jensen, B.B., Finster, K. and Pedersen, K. (2006). Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poultry Science 85, 1151-1164.
Bovee-Oudenhoven, I.M., Ten Bruggencate, S.J., Lettink-Wissink, M.L., Van Der Meer, R. (2003). Dietary fructooligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of Salmonella in rats. Gut 52, 1572-1578.
Buddington, K.K., Donahoo, J.B. and Buddington, R.K. (2002). Dietary oligofructose and inulin protect mice from enteric and systemic pathogens ant tumor inducers. Journal of Nutrition 132, 472-477.
Duncan, S.H., Holtrop, G., Lobley, G.E., Calder, A.G., Stewart, C.S. and Flint, H.J. (2004). Contribution of acetate to butyrate formation by human faecal bacteria. British Journal of Nutrition 91, 915-923.
Durant, J.A., Corrier, D.E. and Ricke, S.C. (2000). Short-chain volatile fatty modulate the expression of the hilA and invF genes of Salmonella Typhimurium. Journal of Food Protection 63, 573-578.
Hinton, M. and Linton, A.H. (1988). Control of Salmonella infections in broiler chickens by the acid treatment of their feed. The Veterinary Record 123, 416-421.
Hinton, M., Linton, A.H., and Perry, F.G. (1985). Control of Salmonella by acid disinfection of chicks’ food. The Veterinary Record 116, 502.
Humblot, C., Bruneau, A., Sutren, M., Lhoste, E.F., Dore, J., Andrieux, C. and Rabot, S. (2005). Brussels sprouts, inulin and fermented milk alter the faecal microbiota of human microbiota-associated rats as shown by PCR-temporal temperature gradient gel electrophoresis using universal, Lactobacillus and Bifidobacterium 16S rRNA gene primers. British Journal of Nutrition 93, 677-684.
Humphrey, T.J. and Lanning, D.G. (1988). The vertical transmission of Salmonella and formic acid treatment of chicken feed. Epidemiology and Infection 100, 43-49.
Iba, A.M. and Berchieri, A. (1995). Studies on the use of a formic acid-propionic acid mixture (Bio-Add™ ) to control experimental Salmonella infection in broiler chickens. Avian Pathology 24, 303-311.
Khan, M. and Katamay, M. (1969). Antagonistic effects of fatty acids against Salmonella in meat and bone meal. Applied Microbiology 17, 402-404.
Lawhon, S.D., Maurer, R., Suyemoto, M and Altier, C. (2002). Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Molecular Microbiology 46, 1451-1464.
Pryde, S.E., Duncan, S.H., Hold, G.L., Stewart, C.S. and Flint, H.J. (2002). The microbiology of butyrate formation in the human colon. FEMS Microbiology Letters 217, 133-139.
Thitaram, S.N., Chung, C.H., Day, D.F., Hinton, A.Jr., Bailey, J.S., Siragusa, G.R. (2005). Isomaltooligosaccharides increases cecal Bifidobacterium population in young broiler chickens. Poultry Science 84, 998-1003.
Thompson, J.L. and Hinton, M. (1997). Antibacterial activity of formic and propionic acids in the diet of hens on Salmonellas in the crop. British Poultry Science 38, 59-65.
Thormar, H., Hilmarsson, H. and Bersson, G. (2006). Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Applied and Environmental Microbiology 72, 522-526.
Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Hautefort, I., Thompson, A., Hinton, J.C. and Van Immerseel, F. (2006). Butyrate specifically down-regulates Salmonella pathogenicity island I gene expression. Applied and Environmental Microbiology 72, 946-949.
MacLeod, H. (2002). Practical experience of feed decontamination from breeder’s perspective. Lohmann Information International 26, 7-8.
Silva, A.M., Barbosa, F.H., Duarte, R., Vieira, L.Q., Arantes, R.M. and Nicoli, J.R. (2004). Effect of Bifidobacterium longum ingestion on experimental salmonellosis in mice. Journal of Applied Microbiology 97, 29-37.
Van Immerseel, F., De Buck, J., Meulemans, G., Pasmans, F., Velge, P., Bottreau, E., Haesebrouck, F. and Ducatelle, R. (2003) Invasion of Salmonella Enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids, International Journal of Food Microbiology, 85, 237 – 248.
Van Immerseel, F., De Buck, J., Boyen, F., Bohez, L., Pasmans, F., Volf, J., Sevcik, M., Rychlik, I., Haesebrouck, F. and Ducatelle, R. (2004a). Medium-chain fatty acids decrease colonization and invasion shortly after infection with Salmonella Enteritidis in chickens through hilA suppression. Applied and Environmental Microbiology 70, 3582-3587.
Van Immerseel, F., Fievez, V., De Buck, J., Pasmans, F., Martel, A., Haesebrouck, F. and Ducatelle, R. (2004b). Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella Enteritidis in young chickens. Poultry Science 83, 69-74.
Van Immerseel, F., Boyen, F., Gantois, I., Timbermont, L., Bohez, L., Pasmans, F., Haesebrouck, F. and Ducatelle, R. (2005). Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poultry Science 84, 1851-1856.










