Salmonella is a widespread problem in poultry farming worldwide. In addition to primary pathogenic salmonella, which can cause high losses in various poultry species, salmonella play a particularly important role as a zoonotic agent, i.e. a disease that can be transmitted from animals to humans.
According to the latest EFSA report, salmonellosis is the number two bacterial zoonosis in Europe (Zoonoses Report 2018)Therefore, the poultry industry has a special responsibility to prevent the introduction of salmonella into the flocks and thus minimise the risk of transmission to humans.

Salmonella are rod-shaped bacteria from the Enterobacteriaceae family that can cause febrile gastrointestinal infections in humans and animals, among other things. Only two species exist, which are divided into over 2,600 salmonella serovars.
Salmonella bongori is mainly found in reptiles. In warm-blooded animals, such as mammals and birds, Salmonella enterica is primarily found. Eight subspecies are distinguished, whereby almost all infections in birds and mammals are caused by the subspecies Salmonella enterica subsp. enterica.
Within this subspecies, there is a further subdivision into so-called salmonella serovars (see Table 1). In addition, these serovars can be divided into biovars and phage types.
Table 1. Classification of some serovars according to Kaufmann-White schemez

In the case of salmonellosis in poultry, a distinction is made between infections with host-specific salmonella serovars and non-host-specific salmonella serovars.
While the host-specific serovars S. Gallinarum Biovar Pullorum (Pullorum disease) and S. Gallinarum Biovar Gallinarum (Fowl typhoid) can lead to severe clinical symptoms and sometimes high losses in chickens, non-host-specific salmonella serovars are primarily of great food hygiene and economic importance as causative agents of zoonoses. These include S. Enteritidis, S. Typhimurium, S. Virchow, S. Hadar and S. Infantis. These only rarely cause clinical illness in poultry, but trigger the majority of salmonelloses in humans.
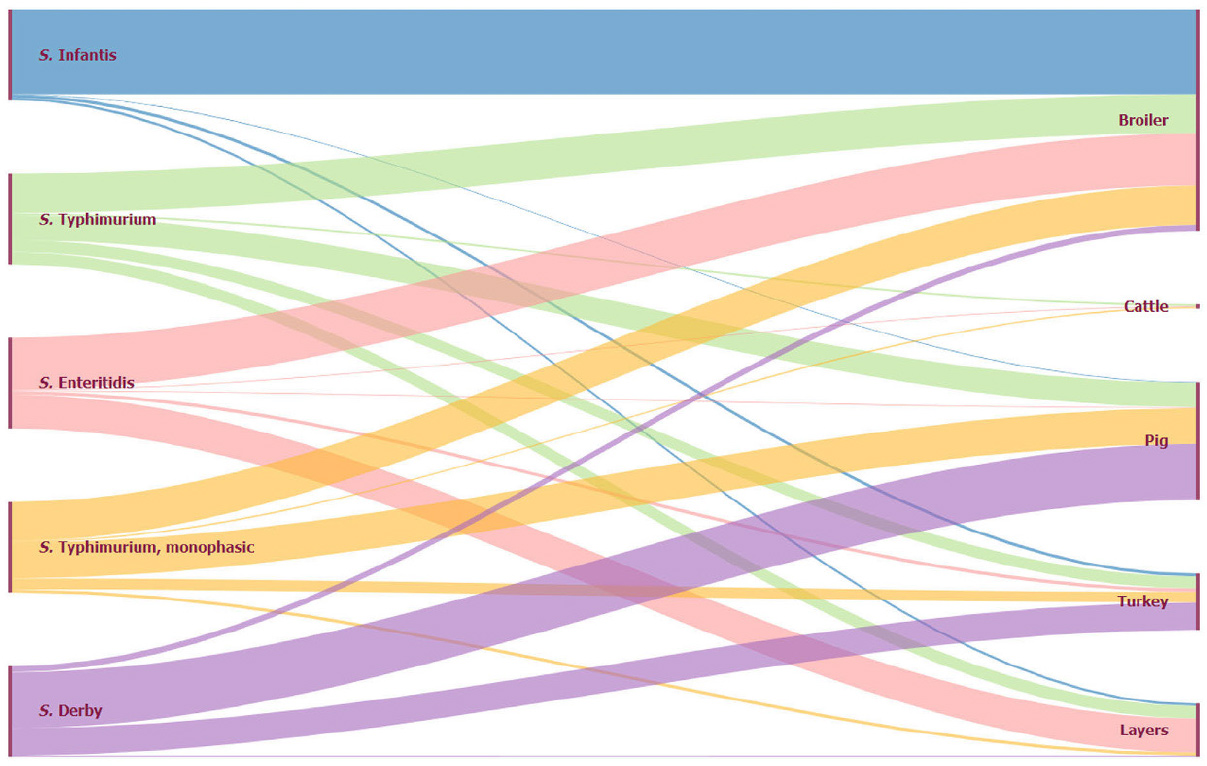
The main sources of infection are (raw) eggs, egg products, insufficiently heated poultry meat, but also pork (see Figure 1).
(Source: Zoonoses Report 2018, https://www.efsa.europa.eu/en/efsajournal/pub/5926)
Figure 1. Animal origin of the five most important human salmonella serovars, EU, 2018
The clinical expression of salmonellosis in poultry depends on:
- The serovar
- The host specificity
- The age of the infected birds
In very young chicks, however, high losses and a chronic disease with joint swelling and respiratory problems may occur. Pathological examination may reveal inflammatory alterations of the navel, heart, appendix and liver. In addition, animals that survive such an early infection are considered lifelong salmonella shedders.

Salmonella Pullorum can already be transmitted vertically, i.e. from infected parents to hatching eggs. Massive hatching loss and weak chicks are the result.
-
- After early infection, the chicks often show chalky white diarrhea (“bacillary white diarrhoea”) and a high mortality of over 50 % (pullorum disease).
-
- Surviving chicks show stunted growth, lameness due to joint inflammation and poorly developed plumage.
- The necropsy shows persistent yolk sacs, swelling of the liver and spleen, inflammation of the appendix and whitish nodules in the heart, liver, pancreas and lungs (“pullorum nodules”).
Older animals may be latently infected with S. Pullorum and show hardly any clinical symptoms. However, performance is impaired, the follicles degenerate and are grey-green in colour and stalked.
Fowl typhoid occurs mainly in adult chickens due to infection with S. Gallinarum. A sudden drop in laying performance and feed intake as well as peracute deaths without preceding clinical symptoms can be observed.
Depending on flock management, age and breed, losses of up to 100% may occur (see Table 2). White hybrid lines are less affected than brown lines.
Table 2. Depending on flock management, age and breed, losses of up to 100% may occur

Direct and indirect methods are used for the diagnosis of salmonella. 

- Bacteriology: direct detection is possible by cultivation on suitable culture media with subsequent identification of the pathogen.
- Molecular biology: salmonella-specific DNA can be detected by PCR
- Commercial serovar-specific PCRs are available for the identification of the serovars S. Enteritidis and S. Typhimurium
Since in some countries vaccination against S. Enteritidis and S. Typhimurium is also carried out with live vaccines, it is important to distinguish between field and vaccine strains
Bacteriological and molecular biological methods are again available for this purpose.
Suitable samples for the direct detection of salmonella are:
- Swabs
- Faeces
- Dust
- Organ samples

Indirect detection of salmonella is done by serological testing of blood samples. These are particularly suitable for screening flocks for latently infected animals that do not show any symptoms but can excrete and transmit salmonella.
- Since antibody detection can sometimes lead to cross-reactions between serovars, a positive result should only be interpreted in combination with direct detection if possible.
- Flocks vaccinated against salmonella can yield positive results in a serological screening.

If salmonella is detected in a flock, this usually has severe economic consequences.
Parent animals can no longer be used for the production of chicks, eggs from laying hens may no longer be sold as grade A and the meat of salmonella-positive animals must be heat-treated after slaughter (national legislation must be considered!).
Even the use of suitable antibiotics cannot guarantee that the treated animals are free of the pathogen.
Herds infected with salmonella therefore continue to be a high risk of infection for humans, which means that such herds often have to be slaughtered prematurely.

Consequently, the focus of salmonella control is on prophylaxis. The introduction of salmonella into poultry flocks must be avoided. Possible carriers are living vectors such as rodents, insects, wild birds, other domestic and farm animals and humans.
Non-living vectors such as equipment, clothing, egg crates and bedding must also be critically considered.
Due to the large number of possible sources of entry, comprehensive hygiene management and a high level of biosecurity are essential (see Figure 2).
Figure 2. Possible sources of entry of salmonella into poultry farming

Special attention must also be paid to feed, through which salmonella can easily be introduced into the farm.
The production process should guarantee freedom from salmonella and care should be taken during storage to ensure that rodents and insects cannot contribute to contamination. Professional rodent and pest control can help minimise the risk.
Another element in the prevention of salmonella introduction is vaccination. In many countries, vaccination of pullets against S. Enteritidis and S. Typhimurium is required by law.
Commercial live vaccines and inactivated vaccines are available. The basic vaccination of pullets is usually carried out by administering live attenuated vaccines twice or preferably three times via the drinking water. 

It is important to ensure that the first vaccination is carried out as early as possible. The first vaccination should be carried out before the first contact with a field salmonelle, so that the full effect of the vaccine can be achieved.
Furthermore, it is possible to boost immunity with inactivated vaccines.

- In some countries, such as France, Denmark, Sweden or Norway, live salmonella vaccines are not licensed.
- In Germany and some other countries, vaccination against S. Gallinarum is prohibited.

Due to the widespread distribution of salmonella in the environment and the large number of possible sources of entry into poultry farming, salmonella will continue to be a considerable problem in poultry production in the future.
Good hygiene management, a high level of biosecurity and thorough vaccination prophylaxis can prevent the introduction of salmonella and thus minimise the risk of human infection.
It is recommended to regularly check one’s own farm for weak points and, if necessary, to involve external advice in order to avoid possible routine blindness. 











 Veterinary
Veterinary 
