Abstract
Bone deformities and fractures have been observed in laying hens since many years and are of concern in the context of modern egg production. One of the most frequently affected bones in laying hens is the sternum (keel bone). A simple method to identify changes of the sternum in live birds is by palpation. In the present study 5869 pure line hens of two different White Leghorn lines (LSL) were palpated twice, at the age of 46 and 70 weeks, to analyze the variation between and within lines. The intra-observer reliability for the palpation score was high (r= 0.82) in both lines. The heritability was moderate in line A, which had 75% deviations (h2= 0.30), and low in line B, which had only 15% deviations (h2= 0.15). The palpation score was not signifcantly correlated with body weight, egg weight and shell breaking strength, but early egg number appears to be negatively correlated with palpation score (rg = -0.24 in line A and -0.54 inline B). The results of the present study indicate that selective breeding may help to reduce the susceptibility to keel bone changes, but at the expense of early egg production.
Keywords
genetics, bone deformity, bone fracture, laying hens, keel boneCo-Authors
W. Icken, F. Kaufmann and M. Schmutz
Introduction
Like all birds, laying hens have the peculiarity of different bone types in their bone system. The cortical and trabecular bone systems give the birds the typical body shape and support their movement, whereas the medullary bone system allows the bird to store calcium for the formation of egg shells. The medullary bone system is subject to a constant assembly and dismantling process. In this process, the osteoblasts store calcium in the medullary bones while the osteoclasts extract calcium for the formation of the egg shell. An imbalance in this assembly and dismantling process of the medullary bone causes the reduction of cortical bone system which may lead to osteoporosis, a complex disease expressed by a decrease in fully mineralized bone structure and an increased risk of fractures and deformities toward the end of the laying period (WHITEHEAD and FLEMING 2000).Litrature
COUCH (1955) described osteoporosis in laying hens, related a high incidence of fractures and mortality to cage management and introduced the term “cage layer fatigue”. GREGORY and WILKINS (1989) described that 29% of caged layers in the United Kingdom suffered one or more bone fractures during their lifetime. The frequency of fractures was found to be higher by GREGORY et al. (1990) in cages (24%) than free range (14%) and aviary systems (10%). Depending on the technical environment and bird management, osteoporosis and broken bones are still observed in modern non-cage systems and call for continued attention. SANDILANDS (2011) reported 53% laying hens with a fracture and 41% with keel bone changes by the end of the laying period in non-cage systems. In general the percentage of hens with fractures at the end of the laying period was highest in free range and aviary systems with 54% and 68%, respectively. To evaluate the causes of fractures and the presence in different housing systems it is important to have a closer look on the age of the fracture. Old (healed) fractures arise during the laying period and are an indication for fractures which are related to the housing environment and are more frequently found in aviary (25%) and free range (12%) than cage systems (5%). The mainly affected bones in aviary systems are the keel bone and clavicle and can be attributed to crashes within the housing system (GREGORY et al. 1990). In contrast, fresh fractures are typically acquired during the depopulation and slaughtering process. According to GREGORY et al. (1994), 17% of the hens at the slaughterhouse had old fractures which have arisen during the time in the cage and 10% new fractures due to the loading and unloading process.As pointed out by FLEMING et al. (1994), movement promotes the development and prevents the loss of structural bone tissue. Especially the possibility of flying in perchery systems, with perches on different levels, increases the breaking strength of the humerus compared to aviary and cage systems. The existence of genetic variation and the possibility to breed for increasing bone strength have been shown by BISHOP et al. (2010). From a commercial White Leghorn line, they developed two sublines by single trait selection in opposite direction on an index for bone quality and reduced the rate of fractures signifcantly within three generations. The estimated heritability for their bone quality index was h2= 0.4, the genetic correlation of the bone quality index with the presence of fractures was rg = -0.9. Within 3 generations the incidence of fractures between the groups high and low bone quality index was reduced by the factor six. The genetic correlation between the bone quality index and the total number of fractures was rg = -0.64.
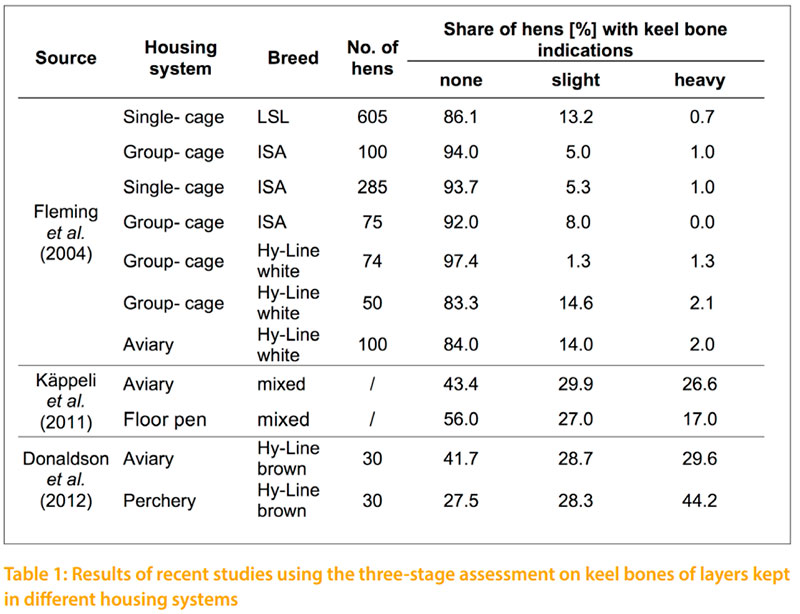
The current most common method for the subjective assessment of bone changes in layers is the keel bone palpation. Changes of the keel bone have to differentiate in fractures and deformations. Fractures are characterized by sharp edges or by an offset in the carina. They can usually be identifed by palpation as callus tissue. While fractures are an obvious defect in the carina, CASEY-TROTT et al. (2015) describes deformations as a deviation from the normal straight two-dimensional line of the carina, which is not due to a fracture. Other authors describe the deformation as a curved sternum or as an S-shaped sternum (FLEMING et al. 2004; HABIG and DISTL 2013). Two different palpation methods are being used. The “Simplifed Keel Assessment Protocol” (SKAP) described by CASEY-TROTT et al. (2015) only records whether fractures and deformations are noticed, on a yes-no scale. This method is particularly easy to use and allows the comparison of different housing systems. In the study of CASEY-TROTT et al. (2015) 202 Lohmann LSL Lite hens from different housing systems were investigated at the age of 68 to 81 weeks by the SKAP procedure. The results show that 46% of the hens had a deformation and 52% had a fracture. A proportion of 37% of the hens showed neither a deformation nor a fracture. On the other hand, 35% of the hens had a fracture and a deformation. A more convenient method is the keel bone palpation as described by SCHOLZ et al. (2008) and KÄPPELI et al. (2011). In this method a distinction is made between a severe change involving a fracture, a slight change, and no change in the sternum. Table 1 shows an overview of various studies using the three-stage assessment on keel bones. According to DONALDSON et al. (2012), there is no link between the provision of higher perches, such as those found in perchery systems, and the occurrence of keel bone damages. KÄPPELI et al. (2011) show a higher incidence of keel bone damages in aviary systems. A general deterioration of the palpation score is expected toward the end of the laying period (FLEMING et al. 2004; DONALDSON et al. 2012; HABIG and DISTL 2013). According to BISHOP et al. (2010), there is a relation to the body weight and the occurrence of keel bone damages (rg = -0.25). A disadvantage of the palpation method is the insufcient repeatability, especially in the case of untrained persons with little experience (CASEYTROTT et al. 2015). In a study of KÄPPELI et al. (2011), the repeatability of the palpation decreased from the frst day with r= 0.95 to 0.79 after 12 weeks. The simplest method to verify the true occurrence of fractures is a visual examination of post-slaughter fractures following the palpation. The accordance between the identifed fractures by palpation and fractures shown at the slaughter house was 84%. The reliability for the identifcation of deformities was 91% (CASEY-TROTT et al. 2015). SCHOLZ et al. (2008) report that error rates of over 51% are possible between the fractures recorded by palpation and the true fractures identifed after slaughter.
Animals and Statistical Methods
In this study a total of 5869 white pure line hens of the LSL breeding program were scored twice by keel bone palpation. Two Lines, line A (n= 2979) and line B (n= 2890), were investigated under comparable conditions on the same farm, in a single bird cage with a round metal perch. The pedigree structure was 90 sires of each line, with 35 daughters per sire in line A and 33 daughters per sire in line B and 4 daughters per dam in both lines. The birds were reared together in a floor barn until 18 weeks of age. After rearing with a day length of 9 hours, the light was gradually increased to 15 hours per day. The hens were fed ad libitum in a two phases: phase 1 from 19 to 45 weeks of age, with 3.9% calcium; phase 2 from 46 weeks until depopulation at the age of 72 weeks, with 4.2% calcium content.The frst palpation was conducted at 46 weeks, the second one at 70 weeks of age. For the palpation the hen is fxed with the left hand by the wings, while the right hand is palpating the keel bone. The direction for the palpation is from cranial to caudal along the carina sterni. The evaluation included the difference of the carina from ventral against dorsal and the lateral shift out of the strait line. The keel bone evaluation was done in a modifed scheme according to SCHOLZ et al. (2008). A four stage assessment was used. If there was no change in the keel the score 4 was given. If there was a small drift in the keel, score 3. By heavier drifts in the keel the score 2 and if there was a fracture in the keel, the score 1 was given. Only the score of the worst incidence was given each time. The palpation was done by one person. The intra-observer reliability was estimated by a two time palpation of 125 birds in both Lines.
The statistical analysis was made with the software SAS 9.3. Correlation between the palpations was estimated by SpearmanRho. Differences were tested for statistical signifcance by the Mann-Whitney U-Test. The genetic variances and covariances were estimated in a mean value mode by the restricted maximum likelihood method (REML) with the computer software VCE 4 (GROENEVELD 1998). This model was used to assess the heritability of the palpation score and genetic correlations with other traits of economic interest: feed intake, body weight, early laying performance, laying performance at peak, egg weight and shell breaking strength.
Yijkl = μ + Hi + Sj + ak + eijkl Yijkl = dependent variable μ = total average value Hi = fxed effect house Sj = fxed effect of hatch week ak = random animal effect eijkl = effect of error
Results and Discussion
The estimated intra-observer reliability of the keel bone palpation was r= 0.82 in this study. In the study of STRATMANN et al. (2015) an intra-observer reliability of r= 0.7 and an inter-observer reliability of r= 0.54 were estimated. The inter-observer reliability is important when the palpation is done by more than one person, which is necessary in large groups of hens. In subjective traits like the palpation, the validation and repeatability of the measurement is a serious challenge. The slight difference between a small and a heavier deformity in the keel bone is sometimes not easy to distinguish. The identifcation of fractures over sharp edges or callus tissue is often even more difcult. The reliability and inter-observer reliability should be improved by teaching the people in palpation and compare their palpation experiences. The evaluation of the validity has to be carried out from time to time by post mortem examinations (CASEY-TROTT et al. 2015)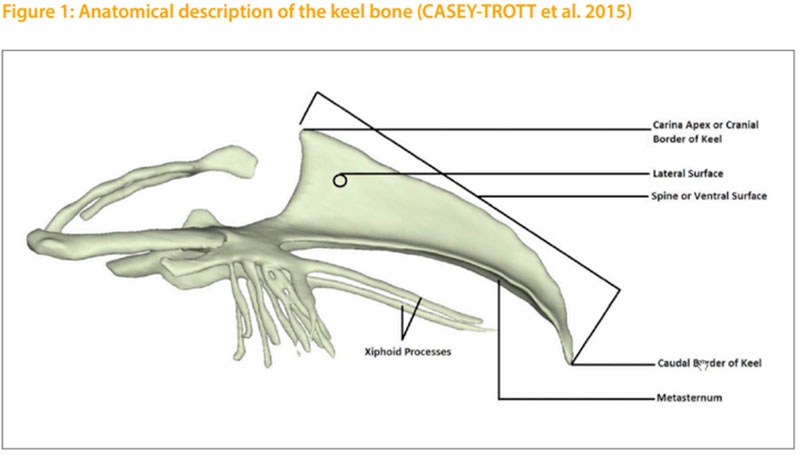
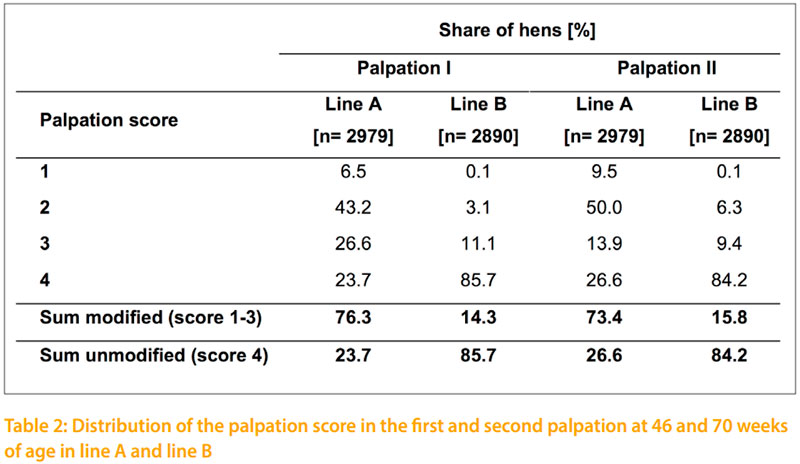
The results of the conducted palpation of this study at 46 and 70 weeks of age are shown in table 2. At both palpation dates there is a much higher incidence of total indication in line A compared to line B. The incidence of fractures in line A increased from 6.5% at 46 weeks to 9.5% at 70 weeks of age. In line B fractures were negligible with 0.1%. Therefore it is important which genotype is used in other approaches. Layers of line A could be used as sentinel birds to take a closer look at different housing equipment for pullets and laying hens which are likely to affect the development of such deviations.
In line A the proportion of hens with and without an indication in the keel bone is nearly the same at the frst and second date of palpation. At 46 weeks of age 76.3% of the hens of line A and 14.3% of line B have a keel bone indication. In the second palpation at 70 weeks of age 73.4% and 15.8% respectively show indications. The correlation between the two palpation dates is rp = +0.8. In a detailed comparison of the two palpation dates it becomes obvious, that the number of fractures and strong deformities increase by time which is in accordance to FLEMING et al. (2004), DONALDSON et al. (2012) and HABIG and DISTL (2013). At 70 weeks of age FLEMING et al. (2004) recorded for 13.2 % of the tested LSL layers a slight change in the keel bone whereas only 0.7 % of the tested layers showed a heavy change in the keel bone. Therefore, the main percentage of LSL layers (86.1%) show no keel bone indication. This coincides with the data of line B from the present study that shows intact keel bones for 84.2% of the tested white layers. The other investigated breeds ISA and Hy Line of FLEMING et al. (2004) obtained equal proportions of keel bones with and without indications like the line B in the current study. Similar results on keel bone indications as shown for line A in this study with 73.4 % are published by KÄPPELI et al. (2011) and DONALDSON et al. (2012) for brown layers in floor and aviary systems at the late stage of production. But KÄPPELI et al. (2011) considers that a comparison on keel bone indications of layers tested in different housing systems is only comparable to a limited extent. Furthermore, KÄPPELI et al. (2011) and DONALDSON et al. (2012) investigated keel bone deformations on brown layers that show due to RICZU et al. (2004) and HABIG and DISTL (2013) a higher bone strength than white layers.
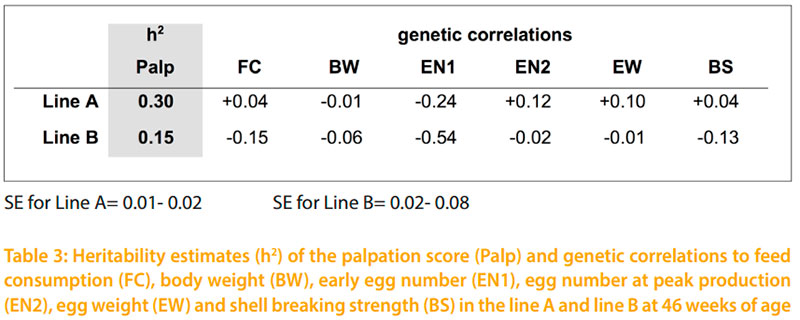
The difference in keel bone indications between line A and line B is at both palpation dates signifcant (p -0.05). Considering the same environment and the same palpating person of both lines, this difference is also due to genetic aspects. The possibility to select on better bones and the chance to reduce the incidence of fractures has been shown by BISHOP et al.(2000). The authors estimated a heritability of h2= 0.4 for their bone quality index. In the present study, the estimated heritability for the keel bone palpation score is h2=0.30 for line A. The lower variance within the palpation score in line B leads to the lower heritability of h2= 0.15 for line B. However, it is planned to continue palpation in the tested lines. Therefore, repeated measurements and additional family information will increase the reliability of the estimated breeding values in the future. The emphasized relationship of BISHOP et al. (2010) between body weight and keel bone changes could not be verifed with this study. The genetic correlation between the individual body weight and palpation score of both lines are rg = -0.01 and -0.06. Even the shell breaking strength and egg weight is due to low genetic correlations not affected by the keel bone status. As shown in table 3 only the early egg number would be affected by selection on better keel bones. The negative genetic correlations of rg = -0.24 in line A and rg = -0.54 in line B would especially in line B decrease the early egg number.
The negative correlation between early egg production and palpation score indicates that late maturing hens are less likely to develop bone defects, which could actually be an advantage in terms of lifetime performance. To assess the usefulness of the palpation score as a novel trait in commercial breeding programs for laying hens, it would be desirable to determine the breeding values for bone quality of all selected male line males and collect data on bone quality of their pedigreed crossline daughters under a range of commercial conditions. This data could be used in the progeny generation to simulate a selection to answer the question, how much progress could be achieved in one generation depending on the relative emphasis of bone quality.
Conclusion
The presence of bone fractures and deformities in different husbandry systems has been documented and discussed in the literature by several authors who agree that this is a multifactorial problem. A decrease of bone changes can only be achieved by an improvement of the housing system, feed composition and the laying hen itself. The shown influence of genotype in this study has to be considered in other approaches. The tested keel bone palpation is an easy and readily implemented method to determine bone changes in layers. Further investigation is needed to fgure out the inter-observer reliability of the keel bone palpation. The option to breed for better bone strength has been shown by BISHOP et al. (2010). Considering the moderate heritability of h²= 0.30 and h²= 0.15 for both tested lines in the shown study, an improvement of the layer keel bone status by selection seems to be possible. The estimated genetic correlations indicate no effect on the egg weight, shell breaking strength and body weight. Regarding the tested traits, only the early egg number would be negatively influenced by selection for better keel bones.

References
BISHOP, S. C., FLEMING, R. H., MCCORMACK, H. A., FLOCK, D. K., WHITEHEAD, C. C. (2000) The inheritance of bone characteristics affecting osteoporosis in laying hens. British Poultry Science 41: 33- 40.CASEY-TROTT, T., HEERKENS, J. L. T., PETRIK, M., REGMI, P., SCHRADER, L., TOSCANO, M. J., WIDOWSKI, T. (2015) Methods for assessment of keel bone damage in poultry. Poultry Science 94: 2239- 2350.
COUCH, F. R. (1955) Cage layer fatigue. Feed Age 5: 55- 57.
DONALDSON, C. J., BALL, M. E. E., O´CONNELL, N. E. (2012) Aerial perches and free-range laying hens: The effect of access to aerial perches and of individual bird parameters on keel bone injuries in commercial free-range laying hens. Poultry Science 91: 304- 315.
FLEMING, R. H., WHITEHEAD, C. C., ALVEY, D., GREGORY, N. G., WILKINS, L. J. (1994) Bone structure and breaking strength in laying hens housed in different husbandry systems. British Poultry Science 35: 651- 662.
FLEMING, R. H., MCCORMACK, H. A., MCTEIR, L., WHITEHEAD, C. C. (2004) Incidence, pathology and prevention of keel bone deformities in the laying hen. British Poultry Science 45: 320- 330.
GREGORY, N. G., WILKINS, L. J. (1989) Broken bones in domestic fowl: Handling and processing damage in end- of- lay battery hens. British Poultry Science 30: 555- 562.
GREGORY, N. G., WILKINS, L. J., ELEPERUMA, S. D., BALLANTYNE, A. J., OVERFIELD, N. D. (1990) Broken bones in domestic fowls: effect of husbandry system and stunning method in end- of- lay hens. British Poultry Science 31: 58- 69.
GREGORY, N. G., WILKINS, L. J., KNOWLES, T. G., SORENSEN, P., VANNIEKERK, T. (1994) Incidence of bone fractures in European layers. Proc. 9th European Poultry Conference Vol. II, Glasgow, UK: 126- 128.
GROENEVELD, E. (1998) VCE 4, 1.3. User`s Guide and Reference Manual.
HABIG, C., DISTL, O. (2013) Evaluation of bone strength, keel bone status, plumage condition and egg quality of two layer lines kept in small group housing systems. British Poultry Science 54: 413- 424.
KÄPPELI, S., GEBHARDT- HENRICH, S. G., FRÖHLICH, E, PFULG, A, STOFFEL, M. H. (2011) Prevalence of keel bone deformities in Swiss laying hens. British Poultry Science 52: 531- 536.
NEWMAN, S., LEESON, S. (1998) Effect of housing birds in cages or aviary system on bone characteristcs. Poultry Science 77: 1492- 1496.
RICZU, C. M., SAUNDERS- BLADES, J. L., YNGVESSON, A. K., ROBINSON, F. E., KORVER, D. R. (2004) End-of-cycle bone quality in white- and brown- egg laying hens. Poultry Science 83: 375- 383.
SANDILANDS, V. (2011) The laying hen and bone fractures. The Veterinary Record 169: 411- 412.
SCHOLZ, B., RÖNCHEN, S., HAMANN, H., SÜRIE, C., NEUMANN, U., KAMPHUES, J., DISTL, O. (2008) Evaluation of bone strength, keel bone deformity and egg quality of laying hens housed in small group housing systems and furnished cages in comparison to an aviary housing system. Archiv für Tierzucht 51: 179- 186.
STRATMANN, A., FRÖHLICH E. K. F., GEBHARDT- HENRICH, S. G, HARLANDERMATAUSCHEK, A., WÜRBEL, H., TOSCANO, M. J. (2015) Modifcation of aviary design reduces incidence of falls, collisions and keel bone damage in laying hens. Appl. Behav. Sci. 165: 112- 123.
WHITEHEAD, C. C., FLEMING, R. H. (2000) Osteoporosis in Cage Layers. Poultry Science 79: 1033- 1041.










